Abstract
Background
Axillary surgery after neoadjuvant chemotherapy (NAC) is becoming less extensive. We evaluated the evolution of axillary surgery after NAC on the multi-institutional I-SPY2 prospective trial.
Methods
We examined annual rates of sentinel lymph node (SLN) surgery with resection of clipped node, if present), axillary lymph node dissection (ALND), and SLN and ALND in patients enrolled in I-SPY2 from January 1, 2011 to December 31, 2021 by clinical N status at diagnosis and pathologic N status at surgery. Cochran-Armitage trend tests were calculated to evaluate patterns over time.
Results
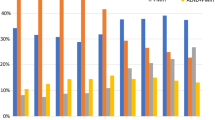
Of 1578 patients, 973 patients (61.7%) had SLN-only, 136 (8.6%) had SLN and ALND, and 469 (29.7%) had ALND-only. In the cN0 group, ALND-only decreased from 20% in 2011 to 6.25% in 2021 (p = 0.0078) and SLN-only increased from 70.0% to 87.5% (p = 0.0020). This was even more striking in patients with clinically node-positive (cN+) disease at diagnosis, where ALND-only decreased from 70.7% to 29.4% (p < 0.0001) and SLN-only significantly increased from 14.6% to 56.5% (p < 0.0001). This change was significant across subtypes (HR−/HER2−, HR+/HER2−, and HER2+). Among pathologically node-positive (pN+) patients after NAC (n = 525) ALND-only decreased from 69.0% to 39.2% (p < 0.0001) and SLN-only increased from 6.9% to 39.2% (p < 0.0001).
Conclusions
Use of ALND after NAC has significantly decreased over the past decade. This is most pronounced in cN+ disease at diagnosis with an increase in the use of SLN surgery after NAC. Additionally, in pN+ disease after NAC, there has been a decrease in use of completion ALND, a practice pattern change that precedes results from clinical trials.




Similar content being viewed by others
References
Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–9.
Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–50.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8.
Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med. 1998;339:941–6.
Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558–66.
Gimbergues P, Abrial C, Durando X, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol. 2008;15:1316–21.
Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2005;23:2694–702.
Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–46.
Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer sentinel node identification and classification after neoadjuvant chemotherapy-systematic review and meta analysis. Acad Radiol. 2009;16:551–63.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy following neoadjuvant chemotherapy in biopsy proven node positive breast cancer: the SN FNAC study. J Clin Oncol. 2013;31:1018.
Classe J-M, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173:343–52.
Piltin M, Boughey J. Axillary management: how has neoadjuvant chemotherapy changed our surgical approach? Curr Breast Cancer Rep. 2022;14:1–7. https://doi.org/10.1007/s12609-022-00442-6.
Boughey JC, Alvarado MD, Lancaster RB, et al. Surgical standards for management of the axilla in breast cancer clinical trials with pathological complete response endpoint. NPJ Breast Cancer. 2018;4:26.
Khan TM, Rossi AJ, Suman V, Haffty B, Hernandez JM, Boughey JC. Is axillary radiation not inferior to axillary dissection for sentinel lymph node-positive breast cancer after neoadjuvant chemotherapy? Ann Surg Oncol. 2022;29:1526–7.
Clinicaltrials.gov. I-SPY TRIAL: Neoadjuvant and personalized adaptive novel agents to treat breast cancer (I-SPY). https://clinicaltrials.gov/ct2/show/NCT01042379. Accessed 20 March 2023.
Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100.
Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375:11–22.
Rugo HS, Olopade OI, DeMichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375:23–34.
van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
DeMichele A, Berry DA, Zujewski J, et al. Developing safety criteria for introducing new agents into neoadjuvant trials. Clin Cancer Res. 2013;19:2817–23.
Yee D, DeMichele AM, et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355–62.
Piltin MA, Hoskin TL, Day CN, Davis J, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27:4795–801.
Nguyen TT, Hoskin TL, Day CN, et al. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25:2596–602.
Mamtani A, Barrio AV, King TA, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? results of a prospective study. Ann Surg Oncol. 2016;23:3467–74.
Srour MK, Tseng J, Luu M, Alban RF, Giuliano AE, Chung A. Patterns in the use of axillary operations for patients with node-positive breast cancer after neoadjuvant chemotherapy: a national cancer database (NCDB) analysis. Ann Surg Oncol. 2019;26:3305–11.
Naffouje SA, Barker V, Lee MC, Hoover SJ, Laronga C. Surgical management of axilla of triple-negative breast cancer in the Z1071 era: a propensity score-matched analysis of the national cancer database. Ann Surg Oncol. 2022;29:2985–97.
Naffouje SA, Sabesan A, Hoover SJ, Lee MC, Laronga C. Surgical management of the axilla of HER2+ breast cancer in the Z1071 era: a propensity-score-matched analysis of the NCDB. Ann Surg Oncol. 2021;28:8777–88.
Funding
National Institutes of Health’s National Cancer Institute (P01 CA210961) and Quantum Leap Healthcare Collaborative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Esserman is an uncompensated board member of the Quantum Leap Healthcare Collaborative, which sponsors the I-SPY TRIAL. She also reports that she is on the Blue Cross Medical Advisory Panel, is paid for travel, and is given an honorarium for her time; she leads an investigator-initiated vaccine trial for high-risk ductal carcinoma in situ, which is funded by Merck through the University of California San Francisco. There are no conflicts related to this manuscript. Dr. Singh reports funding to the institution for the trial through trial sponsors, specifically Foundation for the National Institutes of Health and ACRI. Dr. Tchou is a consultant for BD.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boughey, J.C., Yu, H., Dugan, C.L. et al. Changes in Surgical Management of the Axilla Over 11 Years – Report on More Than 1500 Breast Cancer Patients Treated with Neoadjuvant Chemotherapy on the Prospective I-SPY2 Trial. Ann Surg Oncol 30, 6401–6410 (2023). https://doi.org/10.1245/s10434-023-13759-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13759-y