Abstract
Background
Current success in transplant oncology for select liver tumors, such as hepatocellular carcinoma, has ignited international interest in liver transplantation (LT) as a therapeutic option for nonresectable colorectal liver metastases (CRLM). In the United States, the CRLM LT experience is limited to reports from a handful of centers. This study was designed to summarize donor, recipient, and transplant center characteristics and posttransplant outcomes for the indication of CRLM.
Methods
Adult, primary LT patients listed between December 2017 and March 2022 were identified by using United Network Organ Sharing database. LT for CRLM was identified from variables: “DIAG_OSTXT”; “DGN_OSTXT_TCR”; “DGN2_OSTXT_TCR”; and “MALIG_TY_OSTXT.”
Results
During this study period, 64 patients were listed, and 46 received LT for CRLM in 15 centers. Of 46 patients who underwent LT for CRLM, 26 patients (56.5%) received LTs using living donor LT (LDLT), and 20 patients received LT using deceased donor (DDLT) (43.5%). The median laboratory MELD-Na score at the time of listing was statistically similar between the LDLT and DDLT groups (8 vs. 9, P = 0.14). This persisted at the time of LT (8 vs. 12, P = 0.06). The 1-, 2-, and 3-year, disease-free, survival rates were 75.1, 53.7, and 53.7%. Overall survival rates were 89.0, 60.4, and 60.4%, respectively.
Conclusions
This first comprehensive U.S. analysis of LT for CRLM suggests a burgeoning interest in high-volume U.S. transplant centers. Strategies to optimize patient selection are limited by the scarce oncologic history provided in UNOS data, warranting a separate registry to study LT in CRLM.
Similar content being viewed by others
A liver transplant (LT) is the optimal treatment for patients with select liver tumors, such as hepatocellular carcinoma (HCC), metastatic neuroendocrine tumors (MNETs), or unresectable intra- and hilar cholangiocarcinoma (iCCA/hCCA).1 However, the key to success in these histologies is the appropriate patient selection to maximize the benefit of LT. Until recently, metastatic colorectal cancer to the liver (CRLM) was considered a contraindication for LT therapy due to poor outcomes from the 1980s and 1990s.2 However, this dogma was shaken by Norwegian trials that breathed new life into the concept of LT for unresectable CRLM.
In 2006, the University of Oslo group started a pilot study that reexamined LT in patients with nonresectable CRLM in the era of modern chemotherapy. To date, this group has reported 56 cases of LT for CRLM, and the reported 5-year overall survival (OS) of their two trials are 60 and 83%, respectively. Although most of the patients (44/56) experienced disease recurrence, reported patient survival rates were far superior to those of receiving chemotherapy alone.3,4 Central to the success of the Norwegian protocol was the careful selection of patients who demonstrated sustained disease control, with a latency period before LT, to maximize the likelihood of no extrahepatic disease and favorable tumor biology. Following the Norwegian success, the number of LT for nonresectable CRLM increased worldwide, and the International Hepato-Pancreato-Biliary Association consensus guidelines emerged in 2021.5,6,7
In the United States, the modern-era LT for nonresectable CRLM in 2017, followed shortly by other major centers. Emerging reports from North American experiences have drawn to light similar successes to the outcomes from Oslo.8 However, the current state of LT for CRLM in the United States remains uncharacterized. No contemporary report has described this reemergent indication’s donor, recipient, and center characteristics for LT.
Thus, we set out to understand the trends in LT for CRLM in the United States through an analysis of the United Network Organ Sharing (UNOS) standard transplant and recipient data file. Our objective was to understand trends in LT for CRLM with a focus on donor, recipient, and center characteristics as possible through the UNOS dataset, while understanding inherent limitations in this national registry.
Materials and Methods
Study Population
This study was conducted using the United Network Organ Sharing (UNOS) standard transplant and recipient file data and included listed and transplanted patients between December 2017 and March 2022. Only adult recipients (age > 17 yr) were included. Patients who received multiple organ transplants were excluded from the study. LT for CRLM was identified from variables: “DIAG_OSTXT”; “DGN_OSTXT_TCR”; “DGN2_OSTXT_TCR”; and “MALIG_TY_OSTXT.” The primary outcome of this study was to understand contemporary trends in LT for CRLM, specifically regarding donors, recipients, and performing centers. Therefore, detailed characteristics of listed patients with CRLM were investigated, including patients, donors, and transplant characteristics. The groups consisted of transplants for HCC within high-risk pathological characteristics, transplants for iCCA/hCCA, and transplants for CRLM. HCC with high-risk pathological features was defined as outside Milan Criteria with vascular invasion and/or poorly differentiated components in the pathological specimen. This study was approved by the Institutional Review Board of Stanford University (IRB No. 66782).
Statistical Analysis
Summary statistics were reported as frequencies with percentages, or median values, and interquartile range (IQR). Differences between categorical values were estimated using the chi-square test. In contrast, differences between continuous values were assessed with the Mann-Whitney U test or the Kruskal-Wallis test as appropriate. The Kaplan-Meier method was used to evaluate decease-free survival (DFS) and overall survival (OS) and tested by using the log-rank test. Intraoperative death was included in the survival analyses. Statistical significance was established as P < 0.05.
Results
Overall Characteristics of LT for CRLM
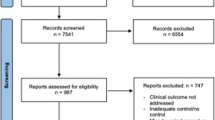
From December 2017 to March 2022, a total of 64 patients were listed for nonresectable CRLM in the United Network for Organ Sharing (UNOS) within respective regions of the United States. Of the 64 patients, 46 (71.9%) received LT, eight (12.5%) were removed from the list, and ten were still on the list as of this analysis. Of 11 UNOS regions, all regions except Region 1 performed LT for CRLM during this period. Nineteen transplant centers listed patients, and 15 centers performed LT for CRLM. Most of them were active liver transplant centers, which performed more than 50 cases per year during the study period (16/19 and 14/15). Five centers performed five or more transplants for CRLM. During the study period, the listed number of CRLM patients increased, and nearly 20 nonresectable CRLM patients were listed in 2020 and 2021 (Fig. 1a). The annual transplanted number of LT for nonresectable CRLM during the study period increased regardless of the introduction of the new allocation system or the COVID-19 pandemic in the winter of 2020 (Fig. 1b). Of 64 patients, 34 patients were listed by centers located different from their home state, and 21 patients were listed by centers that required travel beyond adjacent states (Fig. 1c).
Patient characteristics of those listed, transplanted, and removed from list are summarized in Table 1. The median age of all listed patients was 48 (interquartile range [IQR]: 42-56), and 64.8% were male patients. At the time of listing, the median laboratory MELD-Na was eight (IQR: 6-16). The MELD-Na score of most patients was less than 15 (46/64, 74.2%), and one-fourth were MELD-Na at 6 (18/64, 28.1%), whereas six patients were MELD-Na 25 or higher (9.4%). Of 46 patients who underwent LT for CRLM, 26 patients (56.5%) received LT using living donor LT (LDLT), and 20 patients received LT using deceased donors (DDLT) (43.5%). Of 20 DDLT, two patients (5.0%) received LT using donation after circulatory death, and 15 DDLT were performed after the introduction of the acuity circle policy. The median laboratory MELD-Na score at the time of listing was similar between LDLT and DDLT groups (8 vs. 9, P = 0.14), which was the same at the time of LT (8 vs.12, P = 0.06). Especially in LDLT, 11 of 17 patients were MELD-Na 6, whereas only two DDLT patients were MELD-Na 6 at the time of transplant. Each component of MELD-Na also was compared between the two groups. Although there were no statistical differences, all the lab values of the DDLT group were worse than LDLT, especially the median total bilirubin at the time of LT was 2.7 mg/dL in the DDLT group.
Summary of Deceased Donors Used for LT for Nonresectable CRLM
A detailed summary of the deceased donor is shown in Table 3. The median age of deceased donors was 54 (IQR: 43-59), and most were male (65.4%). Of 20 donors, four donors had a body mass index >35, eight had a history of heavy drinking, and three donors had a history of cancer. Five donors had positive hepatitis B core antibodies, and three donors had positive hepatitis C antibodies, but only one had a positive nucleic acid amplification test. Fourteen donors received a biopsy before organ recovery, two donors had severe macro steatosis, and one patient had grade 3 fibrosis. The median allocation MELD-Na score was 12 (IQR: 8-22). There were 11 livers allocated to the patients with an allocation MELD score <15 (55.0%). Of 20 cases, three (15.0%) were liver-only recoveries, and nine (45.0%) were liver/kidney-only recoveries. Of those, 18 kidneys recovered with liver, and ten kidneys were discarded.
Postoperative Outcomes of LT for CRLM in the US
Ten patients had a recurrence during a median follow-up of 360 days (IQR: 182–672 days), and the earliest tumor recurrence was detected 149 days after LT. Nine patients died after LT: two were intraoperative deaths, one was a COVID-19-related death, and six were as a result of tumor recurrences. The earliest tumor-related death occurred 214 days after LT. The Kaplan-Meier survival curves of DFS and OS of all transplanted patients are shown in Fig. 1d. The 1- and 3-year DFS and OS rates were 75.1% and 53.7%, and 89.0% and 60.4%, respectively. The Kaplan-Meier OS curves are compared between DDLT and LDLT (Fig. 2a). The 1- and 3-year OS of DDLT and LDLT groups were 77.1% and 51.4%, and 100% and 71.4%, respectively (P = 0.049). The postoperative 3-year OS was compared with the survival prognosis of LT for pathological high-risk HCC and iCCA/hCCA performed during the study period. There was no statistical significance difference between OS following LT for nonresectable CRLM and high-risk HCC or iCCA/hCCA.
a Post LT survival outcomes according to donor types. b Post LT survival outcomes according to pre-LT MELD score. c Post LT survival comparison between CRLM and high risk HCC. d Post LT survival comparison between CRLM and intra/hilar cholangiocarcinoma. CRLM Colorectal liver metastases, LT Liver transplant, MELD Model for end-stage liver disease
Discussion
Liver transplantation for colorectal liver metastasis has steadily increased in the United States. Our analysis shows a tenfold increase in listed cases between 2017 and 2022.3 This burst of activity represents a grassroots movement, with five centers from five different UNOS regions performing five or more transplants for CRLM, and 19 centers listing patients for LT for this indication during this study period. However, the 46 performed LT for CRLM is small compared to the total number of transplants performed for primary or secondary liver malignancies over the same period. Similarly, over the study period, an estimated 165,000 new cases of metastatic colorectal cancer were diagnosed.9 Thus, LT for CRLM remains a rare treatment for CRLM in the United States. However, while the rationale for this novel indication stems from encouraging Norwegian trials, we found considerable differences in the recipients, grafts, and transplant center characteristics between the United States and Norway.
In contrast with the population treated in the SECA I and II trials, patients in the United States who received LT for CRLM exhibited two distinct phenotypes. One group, which predominantly received LDLT, underwent LT with a normal hepatic function whose indication is primary oncologic treatment. Conversely, the second cohort of patients who underwent DDLT presented evidence of hepatic dysfunction after extensive oncologic treatment. For example, the median total bilirubin in this second population was 2.7 mg/dL who have burned-out livers—not candidates for further chemotherapy. The inclusion criteria for the SECA II study excluded candidates with baseline total bilirubin greater than two times the upper limit of normal.4 Therefore, the latter population is unique to the United States population, and those findings are consistent with our individual experiences with patients who present to our transplant centers.6
The allografts utilized during this study period were also considerably different compared with the reported Oslo experience. Given the allograft scarcity and lack of UNOS exception points available to patients with CRLM, it is not surprising to see that more than 50% of patients received LDLT. However, available deceased-donor grafts were often marginal, with substantial steatosis, a history of hepatitis or cancer, and overall lower quality than the standard grafts in the Oslo dataset. The high rate of liver-only recoveries further suggests this and the corresponding high discard rate of paired kidneys procured from these marginal donors. Thus, performing DDLTs for nonresectable CRLM in the United States has two challenges related both to donors and recipients. We must contrast the care delivery models between the United States and Norway. SECA I and II were conducted at a single tertiary referral center. The single-payer model in Norway facilitates the standardization of treatment for patients with metastatic colorectal cancer. In this population, the primary tumor is addressed expediently to facilitate future potential therapies. In contrast, the disconnected management of colorectal cancer in the United States often requires acrobatics to address the primary in situ before enrolling patients into LT protocols. Similarly, despite consensus guidelines for LT in CRLM published by the International Hepato-Pancreato-Biliary Association, American centers are at liberty to delineate their protocols.7 It is unclear from the present dataset how stringent each center adheres to these guidelines or other selection criteria codified by prior Norwegian studies.10 In the same breath, our data suggest patients with unresectable CRLM frequently must travel long distances to seek centers that have developed these transplant oncology protocols. While five centers have performed five or more LTs for CRLM, our own experiences suggest the learning curve for managing complexities germane to this population is not insignificant. For example, previous reports have detailed a high utilization of hepatic artery infusion chemotherapy in patients who undergo LDLT for unresectable CRLM.8 In these patients, the native hepatic artery is compromised and requires more complex maneuvers to ensure adequate arterial inflow to the allograft. On the other hand, the concentration of this experience to a few centers is likely to exacerbate transplant disparities and inequities in access, as patients with limited socioeconomic resources are less likely to navigate the hurdles necessary to enter these protocols.11,12,13
Despite differences between recipients, donors, and centers performing LT for CRLM, this analysis of admittedly limited UNOS data suggests results in the United States that mirror early Norwegian trials. With a median follow-up of a year, overall survival at three years was observed to be 60%. Notably, two intraoperative deaths occurred in the DDLT cohort, and one patient died of complications due to COVID-19. For patients who underwent LDLT, the 1- and 3-year OS rate was 100% and 71.4%. Interestingly, recurrence occurred in just 25% of patients at 1 year. This is notable given the 65% and 60% 1-year recurrence rates observed in SECA I and II cohorts, respectively. This again may stem from a population in the United States that is more heavily pretreated and results in liver dysfunction but better disease control and selection. Indeed, subgroup analysis of patients with MELD scores greater than or less than 15 found that patients with elevated MELD scores were at risk of early (intraoperative) death but outlasted their low MELD counterparts in the long run. Finally, when we compare the overall survival of patients with CRLM treated with LT over this period to other complex transplant oncology indications, we found no statistical difference in the observed survival compared to high-risk HCC or iCCA/hCCA. These data support the rationale for including select patients with CRLM who have demonstrated sustained tumor response in future transplant oncology protocols.
Our study is the first to report a recent appraisal of liver transplantation for patients with metastatic colorectal liver metastases in the United States. Our findings suggest a surge of activity in high-volume centers around the country. However, much remains unknown about this population, and as a society, we must now collaborate to hone this interest to steward an increasingly strained liver graft pool appropriately. For starters, while the present dataset represents an excellent tool for transplant-specific outcomes, data on the location of recurrence, preoperative oncologic treatment, and postoperative therapy remains a block box. Thus, we support the call to establish a nationwide registry of patients treated for CRLM with LT to understand these factors and hone our treatment for these patients. Furthermore, we echo the importance of reporting these results to learn the peculiarities of this patient population, which may not fit our previous experiences with other transplant oncology indications. Through critical evaluation of transparent data, we may then consider the appropriateness of incorporating CRLM as a select indication for patients to receive UNOS exception points in the future and provide robust national criteria that all centers may employ to increase access to patients from every walk of life.
Our analysis has several limitations that we must address. First, our observational design is agnostic to individual selection at the transplant center level, which can considerably bias outcomes in a relatively uncommon procedure. Moreover, more than half of the cases included in this study were reported from five centers, and the results of the study were skewed by the practice of those centers. Second, the nascency of this approach limits our follow-up time. From a statistical standpoint, survival analyses should be halted once the proportion of patients free of an event reaches approximately 10–20%. Therefore, the data used in this study were not mature enough to discuss 3-year survival data.16 In this regard, future studies will better elucidate observed survival than statistical estimates. Finally, while the UNOS dataset is designed to capture transplant-centric outcomes, it falls short of detailed accounting of preoperative oncologic treatment, postoperative treatment, and disease-specific outcomes, such as recurrence patterns. These limitations notwithstanding, our analysis of this national dataset found a considerable increase in LT activity for CRLM with distinct patient, graft, and center characteristics to those reported in international reports. The observed 3-year 60% OS is encouraging; however, we must proceed cautiously and work toward a unified registry and protocol to optimize liver transplantation for patients with unresectable colorectal liver metastases.
References
Sapisochin G, Hibi T, Toso C, et al. Transplant oncology in primary and metastatic liver tumors. Ann Surg. 2021;273(3):483–93. https://doi.org/10.1097/sla.0000000000004071.
Mühlbacher F, Huk I, Steininger R, et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc. 1991;23(1 Pt 2):1567–8.
Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257(5):800–6.
Dueland S, Syversveen T, Solheim JM, et al. Survival following liver transplantation for patients with nonresectable liver-only colorectal metastases. Ann Surg. 2020;271(2):212–8.
Line PD, Ruffolo LI, Toso C, Dueland S, Nadalin S, Hernandez-Alejandro R. Liver transplantation for colorectal liver metastases: What do we need to know? Int J Surg. 2020;82S:87–92.
Chávez-Villa M, Ruffolo LI, Tomiyama K, Hernandez-Alejandro R. Where are we now with liver transplant for colorectal metastasis? Current Transp Rep. 2022. https://doi.org/10.1007/s40472-022-00373-2.
Bonney GK, Chew CA, Lodge P, et al. Liver transplantation for non-resectable colorectal liver metastases: the International Hepato-Pancreato-Biliary Association consensus guidelines. Lancet Gastroenterol Hepatol. 2021;6(11):933–46.
Hernandez-Alejandro R, Ruffolo LI, Sasaki K, et al. Recipient and donor outcomes after living-donor liver transplant for unresectable colorectal liver metastases. JAMA Surg. 2022;157(6):524–30.
Colorectal Cancer—Statistics. Cancer.Net. Published June 25, 2012. Accessed 1 Sept 2022. https://www.cancer.net/cancer-types/colorectal-cancer/statistics
Dueland S, Grut H, Syversveen T, Hagness M, Line PD. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am J Transplant. 2020;20(2):530–7.
Mohamed KA, Ghabril M, Desai A, et al. Neighborhood poverty is associated with failure to be waitlisted and death during liver transplantation evaluation. Liver Transplant. 2022;28(9):1441–53.
Ruffolo LI, Zambrano D, Dale BS, et al. Inferior survival is associated with socioeconomic deprivation in hepatocellular carcinoma. J Surg Res. 2022;279:228–39.
Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354–67.
Hibi T, Rela M, Eason JD et al., Liver transplantation for colorectal and neuroendocrine liver metastases and hepatoblastoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1131–5.
Shah SA, Vagefi PA. Living-donor liver transplant for unresectable colorectal liver metastases-let’s walk, not run. JAMA Surg. 2022;157(6):530–1.
Added Stuart J Pocock, Tim C Clayton, Douglas G Altman. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686–9.
Funding
None.
Author information
Authors and Affiliations
Contributions
Participated in research design; KS, KT, FNA, RHA. Participated in the writing of the paper; KS, LR, MHK, MLM, RHA. Participated in the critical review: All authors. Participated in data analysis: KS, KT, RHA.
Corresponding author
Ethics declarations
Disclosures
The authors declare no conflicts or competing interests as described by Liver Transplantation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasaki, K., Ruffolo, L.I., Kim, M.H. et al. The Current State of Liver Transplantation for Colorectal Liver Metastases in the United States: A Call for Standardized Reporting. Ann Surg Oncol 30, 2769–2777 (2023). https://doi.org/10.1245/s10434-023-13147-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13147-6