Abstract
Background
The optimal threshold of surgical margins for breast malignant phyllodes tumors (MPTs) and the impact of adjuvant chemotherapy and radiotherapy were investigated.
Patients and Methods
We conducted a multicenter nationwide retrospective study of all MPT cases with central pathological review within the French Sarcoma Group. Endpoints were local recurrence-free survival (LRFS), metastasis-free survival (MFS), and overall survival (OS) rates.
Results
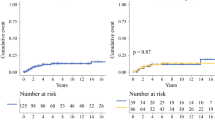
Overall, 212 patients were included in the study. All non-metastatic patients underwent primary surgical treatment, including 58.6% of conservative surgeries. An R0 resection was achieved in 117 patients (59.4%: 26.9% of patients with 1–2 mm margins, 12.2% of patients with 3–7 mm margins, 20.3% of patients with ≥ 8 mm margins). Ninety-four patients (45%) underwent a second surgery (SS) to obtain R0 margins, with a final mastectomy rate of 72.6%. Radiotherapy and chemotherapy were performed in 91 (43.1%) and 23 patients (10.9%), respectively, but were not associated with better outcomes. Mastectomy was significantly associated with better LRFS (p < 0.001). Margins of 0, 1, or 2 mm with SS were associated with better MFS (hazard ratio [HR] 0.3, p = 0.005) and OS (HR 0.32, p = 0.005) compared with margins of 0–1–2 mm without SS. Wider margins (> 8 mm) were not superior to margins of 3–7 mm (3–7 mm vs. > 8 mm; HR 0.81, p = 0.69). Age (HR 2.14, p = 0.038) and tumor necrosis (HR 1.96, p = 0.047) were found to be poor prognostic factors and were associated with MFS.
Conclusions
This study suggests that 3 mm margins are necessary and sufficient for surgical management of MPTs, and emphasizes the importance of SS to obtain clear margins in case of 0–1–2 mm margins. No impact of adjuvant chemotherapy or radiotherapy was detected in this study.




Similar content being viewed by others
References
World Health Organization. WHO classification of tumours of the breast, Fourth Edition. http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4004 (2012). Accessed 25 March 2017.
Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer. 2006;107:2127–33.
Fiks A. Cystosarcoma phyllodes of the mammary gland: Müller’s tumor. For the 180th birthday of Johannes Müller. Virchows Arch A Pathol Anat Histol. 1981;392:1–6.
Lee BJ, Pack GT. Giant intracanalicular myxoma of the breast: the so-called cystosarcoma phyllodes mammae of johannes muller. Ann Surg. 1931;93:250–68.
Desaive P, Betz H. Anatomoclinical aspects of the malignant evolution of a giant intracanalicular fibroadenomyxoma of the breast of Johann Muller phyllodes type [in French]. Presse Med. 1955;63:629–32.
Ward ST, Jewkes AJ, Jones BG, Chaudhri S, Hejmadi RK, Ismail T, et al. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumours of the breast. Int J Surg Lond. 2012;10:527–31.
Confavreux C, Lurkin A, Mitton N, Blondet R, Saba C, Ranchère D, et al. Sarcomas and malignant phyllodes tumours of the breast: a retrospective study. Eur J Cancer Oxf. 2006;42:2715–21.
Kapiris I, Nasiri N, A’Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol. 2001;27:723–30.
Barrio AV, Clark BD, Goldberg JI, Hoque LW, Bernik SF, Flynn LW, et al. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007;14:2961–70.
Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, et al. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016;68:5–21.
Belkacémi Y, Bousquet G, Marsiglia H, Ray-Coquard I, Magné N, Malard Y, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492–500.
Asoglu O, Ugurlu MM, Blanchard K, Grant CS, Reynolds C, Cha SS, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol. 2004;11:1011–7.
Lu Y, Chen Y, Zhu L, Cartwright P, Song E, Jacobs L, et al. Local recurrence of benign, borderline, and malignant phyllodes tumors of the breast: a systematic review and meta-analysis. Ann Surg Oncol. 2019;26:1263–75.
Pezner RD, Schultheiss TE, Paz IB. Malignant phyllodes tumor of the breast: local control rates with surgery alone. Int J Radiat Oncol Biol Phys. 2008;71:710–3.
Onkendi EO, Jimenez RE, Spears GM, Harmsen WS, Ballman KV, Hieken TJ. Surgical treatment of borderline and malignant phyllodes tumors: the effect of the extent of resection and tumor characteristics on patient outcome. Ann Surg Oncol. 2014;21:3304–9.
Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29 Suppl 4:iv267.
Barth RJ. Histologic features predict local recurrence after breast conserving therapy of phyllodes tumors. Breast Cancer Res Treat. 1999;57:291–5.
Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, Souba WW. Surgical management of phyllodes tumors. Arch Surg 1960. 1999;134:487–92. (discussion 492–493).
Mituś J, Reinfuss M, Mituś JW, Jakubowicz J, Blecharz P, Wysocki WM, et al. Malignant phyllodes tumor of the breast: treatment and prognosis. Breast J. 2014;20:639–44.
Jang JH, Choi M-Y, Lee SK, Kim S, Kim J, Lee J, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol. 2012;19:2612–7.
Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998–2009. Ann Surg Oncol. 2014;21:1222–30.
Barth RJ, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16:2288–94.
Kim Y-J, Kim K. Radiation therapy for malignant phyllodes tumor of the breast: an analysis of SEER data. Breast. 2016;32:26–32.
Morales-Vásquez F, Gonzalez-Angulo AM, Broglio K, Lopez-Basave HN, Gallardo D, Hortobagyi GN, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J. 2007;13:551–6.
Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–2.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–7.
Hennigs A, Fuchs V, Sinn H-P, Riedel F, Rauch G, Smetanay K, et al. Do patients after reexcision due to involved or close margins have the same risk of local recurrence as those after one-step breast-conserving surgery? Ann Surg Oncol. 2016;23:1831–7.
Gullett NP, Rizzo M, Johnstone PAS. National surgical patterns of care for primary surgery and axillary staging of phyllodes tumors. Breast J. 2009;15:41–4.
Choi N, Kim K, Shin KH, Kim Y, Moon H-G, Park W, et al. Malignant and borderline phyllodes tumors of the breast: a multicenter study of 362 patients (KROG 16-08). Breast Cancer Res Treat. 2018;171:335–44.
Acknowledgment
The authors are grateful to all the patients and their families. They also thank Dr. Hélène de Forges for substantive editing, Dr. Francoise Ducimetière for data management, and Dr Sebastien Carrere, Dr Carmen Llacer, Dr Aurélie Maran-Gonzalez, Dr. Didier Cupissol, Dr. Alexandre de Nonneville, Dr. Maud Toulmonde, Dr Nicolas Isambert and Dr Pascale Dubray-Longeras for patient inclusions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
Mathias Neron, Christophe Sajous, Simon Thezenas, Sophie Piperno-Neumann, Fabien Reyal, Marick Laé, Camille Chakiba, Audrey Michot, Nicolas Penel, Charles Honoré, Clémentine Owen, François Bertucci, Sébastien Salas, Esma Saada-Bouzid, Thibaud Valentin, Emmanuelle Bompas, Mehdi Brahmi, Isabelle Ray-Coquard, Jean-Yves Blay, and Nelly Firmin have no conflicts of interest to declare for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neron, M., Sajous, C., Thezenas, S. et al. Surgical Margins and Adjuvant Therapies in Malignant Phyllodes Tumors of the Breast: A Multicenter Retrospective Study. Ann Surg Oncol 27, 1818–1827 (2020). https://doi.org/10.1245/s10434-020-08217-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08217-y