Abstract
Background
For patients with peritoneal carcinomatosis undergoing cytoreductive surgery with heated intraperitoneal chemotherapy (CRS/HIPEC), incomplete cytoreduction (CCR2/3) confers morbidity without survival benefit. The aim of this study is to identify preoperative factors which predict CCR2/3.
Methods
All patients who underwent curative-intent CRS/HIPEC of low/high-grade appendiceal, colorectal, or peritoneal mesothelioma cancers in the 12-institution US HIPEC Collaborative from 2000 to 2017 were included (n = 2027). The primary aim is to create an incomplete-cytoreduction risk score (ICRS) to predict CCR2/3 CRS utilizing preoperative data. ICRS was created from a randomly selected cohort of 50% of patients (derivation cohort) and verified on the remaining patients (validation cohort).
Results
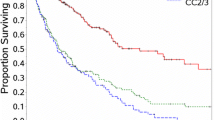
Within our derivation cohort (n = 998), histology was low-grade appendiceal neoplasms in 30%, high-grade appendiceal tumor in 41%, colorectal tumor in 22%, and peritoneal mesothelioma in 8%. CCR0/1 was achieved in 816 patients and CCR 2/3 in 116 patients. On multivariable analysis, preoperative factors associated with incomplete cytoreduction were male gender [odds ratio (OR) 3.4, p = 0.007], presence of ascites (OR 2.8, p = 0.028), cancer antigen (CA)-125 ≥ 40 U/mL (OR 3.4, p = 0.012), and carcinoembryonic antigen (CEA) ≥ 4.2 ng/mL (OR 3.2, p = 0.029). Each preoperative factor was assigned a score of 0 or 1 to form an ICRS from 0 to 4. Scores were grouped as zero (0), low (1–2), or high (3–4). Incidence of CCR2/3 progressively increased by risk group from 1.6% in zero to 13% in low and 39% in high. When ICRS was applied to the validation cohort (n = 1029), this relationship was maintained.
Conclusion
The incomplete cytoreduction risk score incorporates preoperative factors to accurately stratify the risk of CCR2/3 resection in CRS/HIPEC. This score should not be used in isolation, however, to exclude patients from surgery.


Similar content being viewed by others
Notes
Emory University, The Ohio State University, University of California San Diego, University of Cincinnati, City of Hope National Medical Center, Johns Hopkins University, University of Massachusetts, Mayo Clinic, Medical College of Wisconsin, Moffitt Cancer Center, University of Texas MD Anderson Cancer Center, and University of Wisconsin.
References
Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch PH, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21(5):799–806.
Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J. Clinical studies in CRS and HIPEC: Trials, tribulations, and future directions-A systematic review. J Surg Oncol. 2018;117(2):245–9.
Sugarbaker PH, Peritoneal metastases, a frontier for progress. Surg Oncol Clin N Am. 2018;27(3):413–424.
Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010; 28(1):63–8.
Kulu Y, Mueller-Stich B, Buechler MW, Ulrich A. Surgical treatment of peritoneal carcinomatosis: current treatment modalities. Langenbecks Arch Surg. 2014;399(1):41–53.
Foster JM, Sleightholm R, Patel A, Shostrom V, Hall B, Neilsen B, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw Open. 2019;2(1):e186847.
Scaringi S, Leo F, Canonico G, Batignani G, Ficari F, Tonelli F. The role of cytoreductive surgery alone for the treatment of peritoneal carcinomatosis of colorectal origin. A retrospective analysis with regard to multimodal treatments. Hepatogastroenterology. 2009;56(91–92):650–5.
Sugarbaker PH, Schellinx ME, Chang D, Koslowe P, von Meyerfeldt M., Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg. 1996;20(5): 585–91; discussion 592.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H, 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32.
Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20): 3737–43.
Aziz O, Jaradat I, Chakrabarty B, Selvasekar CR, Fulford PE, Saunders MP, et al. Predicting survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendix adenocarcinoma. Dis Colon Rectum. 2018;61(7):795–802.
Elias D, Laurent S, Antoun S, Duvillard P, Ducreux M, Pocard M, et al. Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2003;27(4):407–12.
Kuncewitch M, Levine EA, Shen P, Votanopoulos KI. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendiceal tumors and colorectal adenocarcinomas. Clin Colon Rectal Surg. 2018;31(5):288–294.
Sugarbaker PH, Chang D, Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6(8):727–31.
Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–81.
Baratti D, Kusamura S, Cabras AD, Bertulli R, Hutanu I, Deraco M. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49(15):3140–8.
Deraco M, Casali P, Inglese MG, Baratti D, Pennacchioli E, Bertulli R, et al. Peritoneal mesothelioma treated by induction chemotherapy, cytoreductive surgery, and intraperitoneal hyperthermic perfusion. J Surg Oncol. 2003;83(3):147–53.
Gupta N, Asif S, Gandhi J, Rajpurohit S, Singh S. Role of CRS and HIPEC in appendiceal and colorectal malignancies: Indian experience. Indian J Gastroenterol. 2017;36(2): 126–130.
Chesnais M, Lecuru F, Mimouni M, Ngo C, Fauconnier A, Huchon C. A pre-operative predictive score to evaluate the feasibility of complete cytoreductive surgery in patients with epithelial ovarian cancer. PLoS One. 2017;12(11):e0187245.
Cowan RA, Eriksson AGZ, Jaber SM, Zhou Q, Iasonos A, Zivanovic O, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2017;145(2): 230–5.
Dineen SP, Royal RE, Hughes MS, Sagebiel T, Bhosale P, Overman M, et al. A simplified preoperative assessment predicts complete cytoreduction and outcomes in patients with low-grade mucinous adenocarcinoma of the appendix. Ann Surg Oncol. 2015;22(11):3640–6.
Ghisoni E, Katsaros D, Maggiorotto F, Aglietta M, Vaira M, De Simone M, et al. A predictive score for optimal cytoreduction at interval debulking surgery in epithelial ovarian cancer: a two-centers experience. J Ovarian Res. 2018;11(1):42.
Terzi C, Arslan NC, Canda AE, Peritoneal carcinomatosis of gastrointestinal tumors: where are we now? World J Gastroenterol. 2014;20(39):14371–80.
Baratti D, Kusamura S, Martinetti A, Seregni E, Laterza B, Oliva DG, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14(8): 2300–8.
Baumgartner JM, et al. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2015;22(5):1716–21.
Delhorme JB, Severac F, Averous G, Glehen O, Passot G, Bakrin N, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg. 2018;105(6):668–676.
Govaerts K, Chandrakumaran K, Carr NJ, Cecil TD, Dayal S, Mohamed F, et al. Single centre guidelines for radiological follow-up based on 775 patients treated by cytoreductive surgery and HIPEC for appendiceal pseudomyxoma peritonei. Eur J Surg Oncol. 2018;44(9): 1371–7.
Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol. 2008;31(1): 49–54.
Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428.
Lam JY, McConnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210(3):424–30.
Loggie BW, Fleming RA, McQuellon RP, Russell GB. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67(10):999–1003.
Solomon D, DeNicola NL, Feferman Y, Bekhor E, Reppucci ML, Feingold D, et al. More synchronous peritoneal disease but longer survival in younger patients with carcinomatosis from colorectal cancer undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2019.
Yan TD, Links M, Xu ZY, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93(10):1270–6.
Alexander‐Sefre F, Chandrakumaran K, Banerjee S, Sexton R, Thomas JM, Cecil T, et al. Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis. 2005;7(4):382–6.
Bruno F, Baratti D, Martinetti A, Morelli D, Sottotetti E, Bonini C, et al. Mesothelin and osteopontin as circulating markers of diffuse malignant peritoneal mesothelioma: a preliminary study. Eur J Surg Oncol. 2018;44(6):792–8.
Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87(4):162–6.
Di Fabio F, Aston W, Mohamed F, Chandrakumaran K, Cecil T, Moran B. Elevated tumour markers are normalized in most patients with pseudomyxoma peritonei 7 days after complete tumour removal. Colorectal Dis. 2015;17(8):698–703.
Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: analysis of 519 patients. Eur J Surg Oncol. 2014;40(5):515–520.
van Ruth S, Hart AA, Bonfrer JM, Verwaal VJ, Zoetmulder FA. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9(10):961–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaidi, M.Y., Lee, R.M., Gamboa, A.C. et al. Preoperative Risk Score for Predicting Incomplete Cytoreduction: A 12-Institution Study from the US HIPEC Collaborative. Ann Surg Oncol 27, 156–164 (2020). https://doi.org/10.1245/s10434-019-07626-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07626-y