Abstract
Background
It is unknown how neoadjuvant treatment schedule affects lymph node count (LNC) and lymph node ratio (LNR) and how these correlate with overall survival (OS) in rectal cancer (RC).
Methods
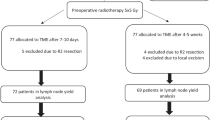
Data were used from the Belgian PROCARE rectal cancer registry on RC patients treated with surgery alone, short-term radiotherapy with immediate surgery (SRT), or chemoradiation with deferred surgery (CRT). The effect of neoadjuvant therapy on LNC was examined using Poisson log-linear analysis. The association of LNC and LNR with overall survival (OS) was studied using Cox proportional hazards models.
Results
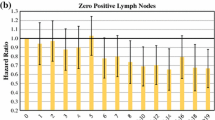
Data from 4037 patients were available. Compared with surgery alone, LNC was reduced by 12.3 % after SRT and by 31.3 % after CRT (p < 0.001). In patients with surgery alone, the probability of finding node-positive disease increased with LNC, while after SRT and CRT no increase was noted for more than 12 and 18 examined nodes, respectively. Per node examined, we found a decrease in hazard of death of 2.7 % after surgery alone and 1.5 % after SRT, but no effect after CRT. In stage III patients, the LNR but not (y)pN stage was significantly correlated with OS regardless of neoadjuvant therapy. Specifically, a LNR > 0.4 was associated with a significantly worse outcome.
Conclusions
Nodal counts are reduced in a schedule-dependent manner by neoadjuvant treatment in RC. After chemoradiation, the LNC does not confer any prognostic information. A LNR of >0.4 is associated with a significantly worse outcome in stage III disease, regardless of neoadjuvant therapy type.



Similar content being viewed by others
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Sobin LH GM, Wittekind C, eds. UICC TNM classification of malignant tumours, 7th ed. Wiley-Blackwell: Oxford, 2010; 310.
Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847–55.
Willaert W, Ceelen W. Extent of surgery in cancer of the colon: is more better? World J Gastroenterol. 2015;21:132–8.
Willaert W, Mareel M, Van De Putte D, Van Nieuwenhove Y, Pattyn P, Ceelen W. Lymphatic spread, nodal count and the extent of lymphadenectomy in cancer of the colon. Cancer Treat Rev. 2014;40:405–13.
Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516.
Miller ED, Robb BW, Cummings OW, Johnstone PA. The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum. 2012;55:1002–7.
Govindarajan A, Gonen M, Weiser MR, et al. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568–73.
Doll D, Gertler R, Maak M, et al. Reduced lymph node yield in rectal carcinoma specimen after neoadjuvant radiochemotherapy has no prognostic relevance. World J Surg. 2009;33:340–7.
Demetter P, Vandendael T, Sempoux C, et al.; Procare. Need for objective and reproducible criteria in histopathological assessment of total mesorectal excision specimens: lessons from a national improvement project. Colorectal Dis. 2013;15:1351–8.
Jegou D, Penninckx F, Vandendael T, Bertrand C, Van Eycken E. Procare. Completeness and registration bias in PROCARE, a Belgian multidisciplinary project on cancer of the rectum with participation on a voluntary basis. Eur J Cancer. 2015; 51:1099–108.
Leonard D, Penninckx F, Kartheuser A, Laenen A, Van Eycken E. Procare. Effect of hospital volume on quality of care and outcome after rectal cancer surgery. Br J Surg. 2014;101:1475–82.
Penninckx F, Kartheuser A, Van de Stadt J, et al. Procare. Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg. 2013;100:1368–75.
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15.
Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–41.
Congdon CC. The destructive effect of radiation on lymphatic tissue. Cancer Res. 1966;26:1211–20.
Elferink MA, Siesling S, Lemmens VE, et al. Variation in lymph node evaluation in rectal cancer: a Dutch nationwide population-based study. Ann Surg Oncol. 2011;18:386–95.
Turner J, Vollmer RT. Lymph nodes in colorectal carcinoma. The Poisson probability paradigm. Am J Clin Pathol. 2006;125:866–72.
Gonen M, Schrag D, Weiser MR. Nodal staging score: a tool to assess adequate staging of node-negative colon cancer. J Clin Oncol. 2009;27:6166–71.
Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267.
Parsons HM, Tuttle TM, Kuntz KM, Begun JW, McGovern PM, Virnig BA. Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA. 2011;306:1089–97.
O’Shea A, Aly O, Parnaby CN, Loudon MA, Samuel LM, Murray GI. Increased lymph node yield in colorectal cancer is not necessarily associated with a greater number of lymph node positive cancers. PLoS One. 2014;9:e104991.
Bhangu A, Kiran RP, Brown G, Goldin R, Tekkis P. Establishing the optimum lymph node yield for diagnosis of stage III rectal cancer. Tech Coloproctol. 2014;18:709–17.
Park IJ, Yu CS, Lim SB, et al. Prognostic implications of the number of retrieved lymph nodes of patients with rectal cancer treated with preoperative chemoradiotherapy. J Gastrointest Surg. 2014;18:1845–51.
Rullier A, Laurent C, Capdepont M, et al. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50.
Tsai CJ, Crane CH, Skibber JM, et al. Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer. 2011;117:3713–22.
Gill A, Brunson A, Lara P, Jr., Khatri V, Semrad TJ. Implications of lymph node retrieval in locoregional rectal cancer treated with chemoradiotherapy: a California Cancer Registry Study. Eur J Surg Oncol. 2015;41:647–52.
Bhatti AB, Akbar A, Hafeez A, et al. Impact of lymph node ratio and number on survival in patients with rectal adenocarcinoma after preoperative chemo radiation. Int J Surg. 2015;13:65–70.
Madbouly KM, Abbas KS, Hussein AM. Metastatic lymph node ratio in stage III rectal carcinoma is a valuable prognostic factor even with less than 12 lymph nodes retrieved: a prospective study. Am J Surg. 2014;207:824–31.
Acknowledgment
The authors thank all of the teams and professionals participating in the PROCARE project. The list of participating centers can be found at www.kankerregister.org under “PROCARE statistics.” PROCARE acknowledges T. Vandendael, data manager, and the BCR for hosting the PROCARE database. PROCARE thanks the Foundation Against Cancer and the National Institute for Disease and Invalidity Assurance for their financial support.
Disclosures
None of the authors have any conflict of interest to declare. No financial or other support was received for the purpose of this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2016_5363_MOESM1_ESM.jpg
Ratio of survival probabilities for LNC = 21 vs LNC = 5 for patients with chemoradiation (n = 2208). Supplementary material 1 (JPEG 98 kb)
Rights and permissions
About this article
Cite this article
Ceelen, W., Willaert, W., Varewyck, M. et al. Effect of Neoadjuvant Radiation Dose and Schedule on Nodal Count and Its Prognostic Impact in Stage II–III Rectal Cancer. Ann Surg Oncol 23, 3899–3906 (2016). https://doi.org/10.1245/s10434-016-5363-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5363-4