Abstract
Background
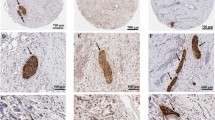
Pancreatic ductal adenocarcinoma (PDAC) frequently invades and migrates along neural tissue, which results in local tumor recurrences, distant metastases, and poor prognosis. We evaluated whether L1 cell adhesion molecule (L1-CAM) and glial cell line-derived neurotrophic factor (GDNF) expression in PDAC correlated with neural invasion and overall survival on a large cohort of previously untreated patients.
Methods
L1-CAM and GDNF were examined by immunohistochemistry in pancreatic cancer tissue samples of 94 cases with PDAC on a tissue microarray. The molecular findings were correlated with pain, clinicopathologic characteristics, and overall survival in these patients.
Results
L1-CAM and GDNF were overexpressed in pancreatic cancer tissues compared with the adjacent normal tissues of pancreas. Positive L1-CAM expression was associated with node involvement (P = 0.007), vascular invasion (P = 0.012), perineural invasion (P = 0.001), and higher degree of pain (P = 0.005). In univariate analysis, tissue expression of L1-CAM was associated with poor survival (hazard ratio, 2.508; 95% confidence interval, 1.551–4.053; P < 0.001), and this was also significant in multivariate analysis (hazard ratio, 2.046; 95% confidence interval, 1.200–3.488; P = 0.009). Positive staining of GDNF, neural invasion, and vascular invasion were all statistically significantly related to unfavorable prognosis.
Conclusions
Enhanced expression of L1-CAM may contribute to the pain syndrome and perineural invasion and may correlate with poor overall survival in human pancreatic cancer.




Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30.
Bergenfeldt M, Moesgaard F, Burcharth F. Curative resection for left-sided pancreatic malignancy. HPB (Oxford). 2006;8:211–5.
Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–62.
Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85.
Ozaki H, Hiraoka T, Mizumoto R, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22.
Takahashi T, Ishikura H, Motohara T, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164–70.
Bockman DE, Buchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219–30.
Nakajima S, Doi R, Toyoda E, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004;10:4125–33.
Kameda K, Shimada H, Ishikawa T, et al. Expression of highly polysialylated neural cell adhesion molecule in pancreatic cancer neural invasive lesion. Cancer Lett. 1999;137:201–7.
Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–81.
Okada Y, Eibl G, Guha S, et al. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–92.
Zhu Z, Friess H, diMola FF, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–28.
Hortsch M. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol Cell Neurosci. 2000;15:1–10.
Salton SR, Richter-Landsberg C, Greene LA, Shelanski ML. Nerve growth factor-inducible large external (NILE) glycoprotein: studies of a central and peripheral neuronal marker. J Neurosci. 1983;3:441–54.
Fransen E, Lemmon V, Van Camp G, et al. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet. 1995;3:273–84.
Kleene R, Yang H, Kutsche M, Schachner M. The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem. 2001;276:21656–63.
Thies A, Schachner M, Moll I, et al. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur J Cancer. 2002;38:1708–16.
Fogel M, Mechtersheimer S, Huszar M, et al. L1 adhesion molecule (CD 171) in development and progression of human malignant melanoma. Cancer Lett. 2003;189:237–47.
Kaifi JT, Reichelt U, Quaas A, et al. L1 is associated with micrometastatic spread and poor outcome in colorectal cancer. Mod Pathol. 2007;20:1183–90.
Gavert N, Sheffer M, Raveh S, et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007;67:7703–12.
Wachowiak R, Fiegel HC, Kaifi JT, et al. L1 is associated with favorable outcome in neuroblastomas in contrast to adult tumors. Ann Surg Oncol. 2007;14:3575–80.
Allory Y, Matsuoka Y, Bazille C, et al. The L1 cell adhesion molecule is induced in renal cancer cells and correlates with metastasis in clear cell carcinomas. Clin Cancer Res. 2005;11:1190–7.
Zecchini S, Bianchi M, Colombo N, et al. The differential role of L1 in ovarian carcinoma and normal ovarian surface epithelium. Cancer Res. 2008;68:1110–8.
Fogel M, Gutwein P, Mechtersheimer S, et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362:869–75.
Issa Y, Nummer D, Seibel T, et al. Enhanced L1CAM expression on pancreatic tumor endothelium mediates selective tumor cell transmigration. J Mol Med. 2009;87:99–112.
Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF). Int J Cancer. 1999;81:67–73.
Iwahashi N, Nagasaka T, Tezel G, et al. Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer. 2002;94:167–74.
Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–300.
Okada Y, Eibl G, Duffy JP, Reber HA, Hines OJ. Glial cell–derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134:293–9.
Sobin LH. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol. 2003;21:19–22.
Koide N, Yamada T, Shibata R, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419–26.
Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73.
Luo J, Zha S, Gage WR, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–6.
Kozarek RA. Endoscopy in the management of malignant obstructive jaundice. Gastroint Endosc Clin N Am. 1996;6:153–76.
Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66.
Nagakawa T, Mori K, Nakano T, et al. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80:619–21.
Lindner J, Rathjen FG, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–30.
Rathjen FG, Rutishauser U. Comparison of two cell surface molecules involved in neural cell adhesion. EMBO J. 1984;3:461–5.
Chang S, Rathjen FG, Raper JA. Neurite outgrowth promoting activity of G4 and its inhibition by monoclonal antibodies. J Neurosci Res. 1990;25:180–6.
Ridder GJ, Klempnauer J. Back pain in patients with ductal pancreatic cancer. Its impact on resectability and prognosis after resection. Scand J Gastroentrol. 1995;30:1216–20.
Dahme M, Bartsch U, Martini R, et al. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–9.
Yamanaka H, Obata K, Kobayashi K, et al. Alteration of the cell adhesion molecule L1 expression in a specific subset of primary afferent neurons contributes to neuropathic pain. Eur J Neurosci. 2007;25:1097–111.
Ohyama R. Clinicopathological studies on carcinoma of the pancreas with special reference to pathological factors affecting the prognosis and lymph node involvement. Nippon Geka Gakkai Zasshi. 1984;85:820–34.
Matsuda M, Nimura Y. Perineural invasion of carcinoma of the head of the pancreas. Jpn J Surg. 1983;84:719–28 (abstract in English).
Gavert N, Conacci-Sorrell M, Gast D, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–42.
Meier F, Busch S, Gast D, et al. The adhesion molecule L1 (CD171) promotes melanoma progression. Int J Cancer. 2006;119:549–55.
Shtfutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–80.
Acknowledgment
We thank Ai-Ying Zeng (University of Fudan) and Fangjun Wan (Ruijin Hospital) for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben, QW., Wang, JC., Liu, J. et al. Positive Expression of L1-CAM is Associated with Perineural Invasion and Poor Outcome in Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 17, 2213–2221 (2010). https://doi.org/10.1245/s10434-010-0955-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-0955-x