Abstract
Background: The aim of the study was (1) to detect candidate genes involved in lymph node metastasis in esophageal cancers and (2) to investigate whether we can estimate and predict occurrence of lymph node metastasis by analyzing artificial neural networks (ANNs) using these gene subsets.
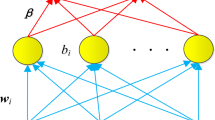
Methods: Twenty-eight primary esophageal squamous cell carcinomas were used. Gene expression profiles of all primary tumors were obtained by cDNA microarray. Lymph node metastasis–related genes were extracted with use of Significance Analysis of Microarrays (SAM). Predictive accuracy for lymph node metastasis was calculated by evaluation of 28 cases by ANNs with leave-one-out cross-n. The results were compared with those of other analyses such as clustering or predictive scoring (LMS).
Results: Our ANN model could predict lymph node metastasis most accurately with 60 clones. The highest predictive accuracy for lymph node metastasis by ANN was 10 of 13 (77%) in newly added cases that were not used for gene selection by SAM and 24 of 28 (86%) in all cases (sensitivity: 15/17, 88%; specificity: 9/11, 82%). Predictive accuracy of LMS was 9 of 13 (69%) in newly added cases and 24 of 28 (86%) in all cases (sensitivity: 17/17, 100%; specificity: 7/11, 67%). It was difficult to extract useful information for the prediction of lymph node metastasis by clustering analysis.
Conclusions: ANN had superior potential in comparison with other methods of analysis for the prediction of lymph node metastasis. This systematic analysis combining SAM with ANN was very useful for the prediction of lymph node metastasis in esophageal cancers and could be applied clinically in the near future.
Similar content being viewed by others
REFERENCES
Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002; 236(2):177–83.
Soehendra N, Binmoeller KF, Bohnacker S, et al. Endoscopic snare mucosectomy in the esophagus without any additional equipment: a simple technique for resection of flat early cancer. Endoscopy 1997; 29(5):380–3.
Nemoto K, Ogawa Y, Matsushita H, et al. A pilot study of radiation therapy combined with daily low-dose cisplatin for esophageal cancer. Oncol Rep 2001; 8(4):785–9.
Gao XS, Qiao XY, Yang XR, et al. Late course accelerated hyperfractionation radiotherapy concomitant with cisplatin in patients with esophageal carcinoma. Oncol Rep 2002; 9(4):767–72.
Shimada Y, Imamura M, Watanabe G, et al. Prognostic factors of oesophageal squamous cell carcinoma from the perspective of molecular biology. Br J Cancer 1999; 80(8):1281–8.
Fukuda M, Hirata K, Natori H. Endoscopic ultrasonography of the esophagus. World J Surg 2000; 24(2):216–26.
Himeno S, Yasuda S, Shimada H, et al. Evaluation of esophageal cancer by positron emission tomography. Jpn J Clin Oncol 2002; 32(9):340–6.
Ross DT, Scherf U, Eisen MB, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 2000; 24(3):227–35.
Kan T, Shimada Y, Sato F, et al. Gene expression profiling in human esophageal cancers using cDNA microarray. Biochem Biophys Res Commun 2001; 286(4):792–801.
Rumelhart DE, Hinton GE, Williams RJ, et al. Learning representations by back-propagating errors. Nature 1986; 323:533–6.
Xu Y, Selaru FM, Yin J, et al. Artificial neural networks and gene filtering distinguish between global gene expression profiles of Barrett’s esophagus and esophageal cancer. Cancer Res 2002; 62(12):3493–7.
Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001; 98(9):5116–21.
Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998; 95(25):14863–8.
Kihara C, Tsunoda T, Tanaka T, et al. Prediction of sensitivity of esophageal tumors to adjuvant chemotherapy by cDNA microarray analysis of gene-expression profiles. Cancer Res 2001; 61(17):6474–9.
Nagayama S, Katagiri T, Tsunoda T, et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res 2002; 62(20):5859–66.
Sato F, Shimada Y, Li Z, et al. Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg 2001; 88(3):426–32.
Selaru FM, Zou T, Xu Y, et al. Global gene expression profiling in Barrett’s esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene 2002; 21(3):475–8.
Yang YH, Dudoit S, Luu P, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 2002; 30(4):e15.
Wittwer CT, Ririe KM, Andrew RV, et al. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques 1997; 22(1):176–81.
Rychlik W, Rhoads RE. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res 1989; 17(21):8543–51.
Nishimaki T, Tanaka O, Suzuki T, et al. Tumor spread in superficial esophageal cancer: histopathologic basis for rational surgical treatment. World J Surg 1993; 17(6):766–71;discussion, 771–2.
Biagiotti R, Desii C, Vanzi E, Gacci G. Predicting ovarian malignancy: application of artificial neural networks to transvaginal and color Doppler flow US. Radiology 1999; 210(2):399–403.
Marchevsky AM, Patel S, Wiley KJ, et al. Artificial neural networks and logistic regression as tools for prediction of survival in patients with Stages I and II non-small cell lung cancer. Mod Pathol 1998; 11(7):618–25.
Tu JV. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 1996; 49(11):1225–31.
Bottaci L, Drew PJ, Hartley JE, et al. Artificial neural networks applied to outcome prediction for colorectal cancer patients in separate institutions. Lancet 1997; 350(9076):469–72.
Hosch S, Kraus J, Scheunemann P, et al. Malignant potential and cytogenetic characteristics of occult disseminated tumor cells in esophageal cancer. Cancer Res 2000; 60(24):6836–40.
Koyama H, Iwata H, Kuwabara Y, et al. Gelatinolytic activity of matrix metalloproteinase-2 and -9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer 2000; 36(16):2164–70.
Yamashita K, Mori M, Shiraishi T, et al. Clinical significance of matrix metalloproteinase-7 expression in esophageal carcinoma. Clin Cancer Res 2000; 6(3):1169–74.
Sato F, Shimada Y, Watanabe G, et al. Expression of vascular endothelial growth factor, matrix metalloproteinase-9 and E-cadherin in the process of lymph node metastasis in oesophageal cancer. Br J Cancer 1999; 80(9):1366–72.
Etoh T, Inoue H, Yoshikawa Y, et al. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut 2000; 47(1):50–6.
Kadowaki T, Shiozaki H, Inoue M, et al. E-cadherin and alpha-catenin expression in human esophageal cancer. Cancer Res 1994; 54(1):291–6.
Zhou XB, Lu N, Zhang W, et al. [Expression and significance of beta-catenin in esophageal carcinoma]. Ai Zheng 2002; 21(8):877–80.
Nakano S, Baba M, Natsugoe S, et al. Detection of lymph node metastasis using desmoglein 1 expression in superficial esophageal cancer in relation to the endoscopic mucosal resection. Dis Esophagus 1998; 11(3):157–61.
Uchida S, Shimada Y, Watanabe G, et al. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer 1998; 77(10):1704–9.
Noguchi T, Takeno S, Shibata T, et al. VEGF-C expression correlates with histological differentiation and metastasis in squamous cell carcinoma of the esophagus. Oncol Rep 2002; 9(5):995–9.
von Biberstein SE, Spiro JD, Lindquist R, Kreutzer DL. Interleukin-1 receptor antagonist in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 1996; 122(7):751–9.
Dong G, Chen Z, Kato T, Van Waes C. The host environment promotes the constitutive activation of nuclear factor-kappaB and proinflammatory cytokine expression during metastatic tumor progression of murine squamous cell carcinoma. Cancer Res 1999; 59(14):3495–504.
Matar P, Rozados VR, Roggero EA, Scharovsky OG. Lovastatin inhibits tumor growth and metastasis development of a rat fibrosarcoma. Cancer Biother Radiopharm 1998; 13(5):387–93.
Shin MS, Kim HS, Lee SH, et al. Alterations of Fas-pathway genes associated with nodal metastasis in non-small cell lung cancer. Oncogene 2002; 21(26):4129–36.
Silverman RH, Halloum A, Zhou A, et al. Suppression of ovarian carcinoma cell growth in vivo by the interferon-inducible plasma membrane protein, phospholipid scramblase 1. Cancer Res 2002; 62(2):397–402.
Fernandez M, Eng C. The expanding role of PTEN in neoplasia: a molecule for all seasons? [Commentary re: M. A. Davies, et al. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin Cancer Res 2002;8(6):1695–8.] Clin Cancer Res 2002; 8:1904–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kan, T., Shimada, Y., Sato, F. et al. Prediction of Lymph Node Metastasis with Use of Artificial Neural Networks Based on Gene Expression Profiles in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 11, 1070–1078 (2004). https://doi.org/10.1245/ASO.2004.03.007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1245/ASO.2004.03.007