Abstract
Background
Gene mutations in the pathway downstream of epidermal growth factor receptor (EGFR) are considered to induce resistance to anti-EGFR therapy in colorectal cancer (CRC). We recently reported that microRNA-31 (miR-31)-5p may regulate BRAF activation and play a role in the signaling pathway downstream of EGFR in CRC. Therefore, we hypothesized that miR-31-5p can be a useful biomarker for anti-EGFR therapy in CRC.
Methods
We evaluated miR-31-5p expression and gene mutations [KRAS (codon 61 or 146), NRAS (codon 12, 13, or 61), and BRAF (V600E)] in the EGFR downstream pathway in 102 CRC patients harboring KRAS (codon 12 or 13) wild-type who were treated with anti-EGFR therapeutics. Progression-free survival (PFS) and overall survival (OS) were evaluated.
Results
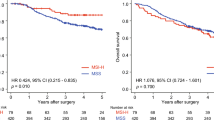
KRAS (codon 61 or 146), NRAS, and BRAF mutations were detected in 6.9, 6.9, and 5.9 % patients, respectively. Compared with CRCs with at least one mutation (n = 20), significantly better PFS (P = 0.0003) but insignificantly better OS were observed in CRCs harboring all wild-type genes (KRAS, NRAS, and BRAF). High miR-31-5p expression was identified in 11 % (n = 11) patients and was significantly associated with shorter PFS (P = 0.003). In CRCs carrying all wild-type genes, high miR-31-5p was associated with shorter PFS (P = 0.027).
Conclusions
High miR-31-5p expression was associated with shorter PFS in patients with CRC treated with anti-EGFR therapeutics. Moreover, in CRCs carrying all wild-type genes, high miR-31-5p was associated with shorter PFS, suggesting that it may be a useful and additional prognostic biomarker for anti-EGFR therapy.


Similar content being viewed by others
References
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34.
Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–21.
De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62.
De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15.
Blanke CD, Goldberg RM, Grothey A, et al. KRAS and colorectal cancer: ethical and pragmatic issues in effecting real-time change in oncology clinical trials and practice. Oncologist. 2011;16:1061–8.
Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12.
Loupakis F, Pollina L, Stasi I, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622–9.
Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–30.
Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–61.
De Roock W, Lambrechts D, Tejpar S. K-ras mutations and cetuximab in colorectal cancer. N Engl J Med. 2009;360:834; author reply 5–6.
Saridaki Z, Tzardi M, Papadaki C, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in ≥2 line cetuximab-based therapy of colorectal cancer patients. PLoS One. 2011;6:e15980.
Sood A, McClain D, Maitra R, et al. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:143–50.
Prenen H, De Schutter J, Jacobs B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–8.
Pentheroudakis G, Kotoula V, De Roock W, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer. 2013;13:49.
Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34.
Devun F, Bousquet G, Biau J, et al. Preclinical study of the DNA repair inhibitor Dbait in combination with chemotherapy in colorectal cancer. J Gastroenterol. 2012;47:266–75.
Wu KL, Huang EY, Jhu EW, et al. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J Gastroenterol. 2013;48:350–9.
Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9.
Zhang Y, Guo J, Li D, et al. Down-regulation of miR-31 expression in gastric cancer tissues and its clinical significance. Med Oncol. 2010;27:685–9.
Bartley AN, Yao H, Barkoh BA, et al. Complex patterns of altered MicroRNA expression during the adenoma-adenocarcinoma sequence for microsatellite-stable colorectal cancer. Clin Cancer Res. 2011;17:7283–93.
Balaguer F, Moreira L, Lozano JJ, et al. Colorectal cancers with microsatellite instability display unique miRNA profiles. Clin Cancer Res. 2011;17:6239–49.
Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–92.
Pagliuca A, Valvo C, Fabrizi E, et al. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2013;32:4806–13.
Arcaroli JJ, Quackenbush KS, Powell RW, et al. Common PIK3CA mutants and a novel 3’ UTR mutation are associated with increased sensitivity to saracatinib. Clin Cancer Res. 2012;18:2704–14.
Valastyan S, Reinhardt F, Benaich N, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–46.
Creighton CJ, Fountain MD, Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–15.
Leidner RS, Ravi L, Leahy P, et al. The microRNAs, MiR-31 and MiR-375, as candidate markers in Barrett’s esophageal carcinogenesis. Genes Chromosom Cancer. 2012;51:473–9.
Wang CJ, Zhou ZG, Wang L, et al. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers. 2009;26:27–34.
Chang KH, Miller N, Kheirelseid EA, et al. MicroRNA signature analysis in colorectal cancer: identification of expression profiles in stage II tumors associated with aggressive disease. Int J Colorectal Dis. 2011;26:1415–22.
Schee K, Boye K, Abrahamsen TW, et al. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC Cancer. 2012;12:505.
Cekaite L, Rantala JK, Bruun J, et al. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868–79.
Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402.
Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–302.
Pichler M, Winter E, Stotz M, et al. Down-regulation of KRAS-interacting miRNA-143 predicts poor prognosis but not response to EGFR-targeted agents in colorectal cancer. Br J Cancer. 2012;106:1826–32.
Zhang W, Winder T, Ning Y, et al. A let-7 microRNA-binding site polymorphism in 3’-untranslated region of KRAS gene predicts response in wild-type KRAS patients with metastatic colorectal cancer treated with cetuximab monotherapy. Ann Oncol. 2011;22:104–9.
Kurokawa K, Tanahashi T, Iima T, et al. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. J Gastroenterol. 2012;47:883–95.
Kalimutho M, Del Vecchio Blanco G, Di Cecilia S, et al. (2011) Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. J Gastroenterol. 46:1391–402.
Iino I, Kikuchi H, Miyazaki S, et al. Effect of miR-122 and its target gene cationic amino acid transporter 1 on colorectal liver metastasis. Cancer Sci. 2013;104:624–30.
Tsuchida A, Ohno S, Wu W, et al. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011;102:2264–71.
Nosho K, Igarashi H, Nojima M, et al. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis. 2014;35:776–83.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Irahara N, Baba Y, Nosho K, et al. NRAS mutations are rare in colorectal cancer. Diagn Mol Pathol. 2010;19:157–63.
Vaughn CP, Zobell SD, Furtado LV, et al. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosom Cancer. 2011;50:307–12.
Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–59.
Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–9.
Manceau G, Imbeaud S, Thiebaut R, et al. Hsa-miR-31-3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. 2014;20:3338–47.
Acknowledgment
The authors thank the pathology departments of Sapporo Medical University Hospital and Keiyukai Sapporo Hospital for providing the tissue specimens. The authors also thank Enago (www.enago.jp) for English language review. This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research [Grant Numbers: 23790800 (to K.N.) and 23390200 (to Y.S.)], A-STEP (Adaptable & Seamless, Technology Transfer Program through Target-driven R&D) (to K.N.), Sapporo Jikeikai Tomoiki Foundation (to K.N.), Takeda Science Foundation (to K.N.), and Daiwa Securities Health Foundation (to H.I.).
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hisayoshi Igarashi, Hiroyoshi Kurihara, Kei Mitsuhashi, and Miki Ito have contributed equally to this work.
Hiroyuki Yamamoto, Katsuhiko Nosho, and Yasuhisa Shinomura have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2014_4264_MOESM2_ESM.tif
Supplementary material 2 Supplementary Figure 1 The scatter diagram of relative expression levels of micorRNA-31-5p and -3p of 102 colorectal cancer (CRC) patients who received anti-EGFR therapy (TIFF 78 kb)
10434_2014_4264_MOESM3_ESM.tif
Supplementary material 3 Supplementary Figure 2. Kaplan–Meier survival curves of patients treated with anti-EGFR therapy according to the mutational status in KRAS, NRAS, and BRAF genes. (a) Overall survival (OS) of patients with at least one mutation in KRAS (codon 61 or 146) or NRAS (codon 12, 13, or 61) versus patients with all wild-type copies of the 2 genes. (b) OS of patients with mutation in BRAF versus patients with wild-type copies of BRAF. (c) OS of patients with at least one mutation in KRAS, NRAS, and BRAF versus all wild-type copies of the 3 genes. (TIFF 117 kb)
10434_2014_4264_MOESM4_ESM.tif
Supplementary material Supplementary Figure 3. Kaplan–Meier survival curves of patients treated with anti-EGFR therapy according to microRNA-31-5p expression. Overall survival of the high-expression group versus the low-expression group 4 (TIFF 60 kb)
10434_2014_4264_MOESM5_ESM.tif
Supplementary material Supplementary Figure 4. Kaplan–Meier survival curves of patients treated with anti-EGFR therapy according to microRNA-31-3p expression. (a) Progression-free survival and (b) overall survival of the high-expression group versus the low-expression group 5 (TIFF 88 kb)
10434_2014_4264_MOESM6_ESM.tif
Supplementary material 6 Supplementary Figure 5. The distribution of relative expression levels of miR-31-5p or -3p of colon cancer cell lines. (TIFF 44 kb)
Rights and permissions
About this article
Cite this article
Igarashi, H., Kurihara, H., Mitsuhashi, K. et al. Association of MicroRNA-31-5p with Clinical Efficacy of Anti-EGFR Therapy in Patients with Metastatic Colorectal Cancer. Ann Surg Oncol 22, 2640–2648 (2015). https://doi.org/10.1245/s10434-014-4264-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4264-7