Abstract
Background
RRAD, a small Ras-related GTPase, is highly expressed in human skeletal muscle, lung, and heart. Although loss of expression of RRAD in breast cancer cells has been reported and it may act as an oncogene, the mechanism of silencing is unknown.
Methods
We examined (1) mRNA expression of RRAD in lung and breast cancer cell lines using RT-PCR and (2) methylation status of lung and breast cancers.
Results
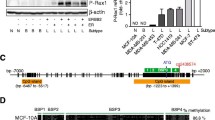
Loss of RRAD expression was found in 14 of 20 (70%) NSCLC cell lines, 11 of 11 (100%) SCLC cell lines, and 8 of 10 (80%) breast cancer cell lines; expression was not affected in normal bronchial and mammary epithelial cells. Treatment of 23 expression-negative cell lines with a demethylating agent restored expression in all cases. We developed a methylation-specific assay from the analysis of bisulfite sequencing of the 5′ region of RRAD in expression-negative and positive cell lines, which resulted in good concordance between methylation and expression. Primary lung and breast cancers showed hypermethylation in 89 of 214 (42%) and 39 of 63 (62%) cases, respectively. RRAD hypermethylation correlated with smoking history and poorer prognosis in lung adenocarcinomas.
Conclusions
We conclude that epigenetic silencing of RRAD is a frequent event in lung and breast cancers, and analysis of it may provide novel opportunities for prognosis and therapy of these cancers.




Similar content being viewed by others
References
Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science 1993; 262:1441–4
Zhu J, Reynet C, Caldwell JS, et al. Characterization of Rad, a new member of Ras/GTPase superfamily, and its regulation by a unique GTPase-activating protein (GAP)-like activity. J Biol Chem 1995; 270:4805–12
Zhu J, Tseng YH, Kantor JD, et al. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci U S A 1999; 96:14911–8
Moyers JS, Bilan PJ, Reynet C, et al. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem 1996; 271:23111–6
Billiard J, Moran RA, Whitley MZ, et al. Transcriptional profiling of human osteoblast differentiation. J Cell Biochem 2003; 89:389–400
Tseng YH, Vicent D, Zhu J, et al. Regulation of growth and tumorigenicity of breast cancer cells by the low molecular weight GTPase Rad and nm23. Cancer Res 2001; 61:2071–9
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3:415–28
Zochbauer-Muller S, Minna JD, Gazdar AF. Aberrant DNA methylation in lung cancer: biological and clinical implications. Oncologist 2002; 7:451–7
Suzuki M, Shigematsu H, Shames DS, et al. DNA methylation-associated inactivation of TGFbeta-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br J Cancer 2005; 93:1029–37
Suzuki M, Toyooka S, Shivapurkar N, et al. Aberrant methylation profile of human malignant mesotheliomas and its relationship to SV40 infection. Oncogene 2005; 24:1302–8
Suzuki M, Hao C, Takahashi T, et al. Aberrant methylation of SPARC in human lung cancers. Br J Cancer 2005; 92:942–8
Suzuki M, Chen H, Shigematsu H, et al. Aberrant methylation: common in thymic carcinomas, rare in thymomas. Oncol Rep 2005; 14:1621–4
Suzuki M, Sunaga N, Shames DS, et al. RNA interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res 2004; 64:3137–43
Suzuki M, Shigematsu H, Takahashi T, et al. Aberrant methylation of Reprimo in lung cancer. Lung Cancer 2005; 47:309–14
Chen H, Suzuki M, Nakamura Y, et al. Aberrant methylation of FBN2 in human non-small cell lung cancer. Lung Cancer 2005; 50:43–9
Kulaeva OI, Draghici S, Tang L, et al. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene 2003; 22:4118–27
Sato S, Nakamura Y, Tsuchiya E. Difference of allelotype between squamous cell carcinoma and adenocarcinoma of the lung. Cancer Res 1994; 54:5652–5
Girard L, Zochbauer-Muller S, Virmani AK, et al. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res 2000; 60:4894–906
Callen DF, Crawford J, Derwas C, et al. Defining regions of loss of heterozygosity of 16q in breast cancer cell lines. Cancer Genet Cytogenet 2002; 133:76–82
Phelps RM, Johnson BE, Ihde DC, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem 1996; 24(Suppl):32–91
Gazdar AF, Kurvari V, Virmani A, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer 1998; 78:766–74
Herrmann BG, Frischauf AM. Isolation of genomic DNA. Methods Enzymol 1987; 152:180–3
Herman JG, Graff JR, Myohanen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996; 93:9821–6
Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol 1987; 196:261–82
Toyooka S, Maruyama R, Toyooka KO, et al. Smoke exposure, histologic type and geography-related differences in the methylation profiles of non-small cell lung cancer. Int J Cancer 2003; 103:153–60
Acknowledgments
This work was supported by grants from the University of Texas Specialized Program of Research Excellence in Lung Cancer (NCI P50CA70907) and Early Detection Research Network (5U01CA8497102), the Gillson Longenbaugh Foundation, and the Emphasis Research Project by expenditure at the discretion of the president of The Chiba University in 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, M., Shigematsu, H., Shames, D.S. et al. Methylation and Gene Silencing of the Ras-Related GTPase Gene in Lung and Breast Cancers. Ann Surg Oncol 14, 1397–1404 (2007). https://doi.org/10.1245/s10434-006-9089-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9089-6