Abstract
Background
Uveal melanoma (UM) has a poor prognosis once liver metastases occur. The melphalan/Hepatic Delivery System (melphalan/HDS) is a drug/device combination used for liver-directed treatment of metastatic UM (mUM) patients. The purpose of the FOCUS study was to assess the efficacy and safety of melphalan/HDS in patients with unresectable mUM.
Methods
Eligible patients with mUM received treatment with melphalan (3.0 mg/kg ideal body weight) once every 6 to 8 weeks for a maximum of six cycles. The primary end point was the objective response rate (ORR). The secondary end points included duration of response (DOR), overall survival (OS), and progression-free survival (PFS).
Results
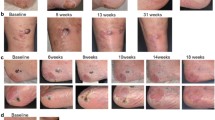
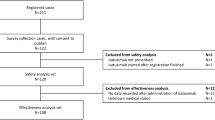
The study enrolled 102 patients with mUM. Treatment was attempted in 95 patients, and 91 patients received treatment. In the treated population (n = 91), the ORR was 36.3 % (95 % confidence interval [CI], 26.44–47.01), including 7.7 % of patients with a complete response. Thus, the study met its primary end point because the lower bound of the 95 % CI for ORR exceeded the upper bound (8.3 %) from the benchmark meta-analysis. The median DOR was 14 months, and the median OS was 20.5 months, with an OS of 80 % at 1 year. The median PFS was 9 months, with a PFS of 65 % at 6 months. The most common serious treatment-emergent adverse events were thrombocytopenia (15.8 %) and neutropenia (10.5 %), treated mostly on an outpatient basis with observation. No treatment-related deaths were observed.
Conclusion
Treatment with melphalan/HDS provides a clinically meaningful response rate and demonstrates a favorable benefit-risk profile in patients with unresectable mUM (study funded by Delcath; ClinicalTrials.gov identifier: NCT02678572; EudraCT no. 2015-000417-44).


Similar content being viewed by others
References
Carvajal RD, Sacco JJ, Jager MJ, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. 2023;20:99–115.
Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019;29:561–8.
Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30:1370–80.
Lane AM, Kim IK, Gragoudas ES. Survival rates in patients after treatment for metastasis from uveal melanoma. JAMA Ophthalmol. 2018;136:981–6.
Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206.
Koch EA, Petzold A, Wessely A, et al. Immune checkpoint blockade for metastatic uveal melanoma: patterns of response and survival according to the presence of hepatic and extrahepatic metastasis. Cancers Basel. 2021;13:3359.
Mignard C, Huvier AD, Gillibert A, et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J Oncol. 2018;2018:1908065.
Najjar YG, Navrazhina K, Ding F, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer. 2020;8:e000331.
Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–53.
Pelster MS, Gruschkus SK, Bassett R, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J Clin Oncol. 2021;39:599–607.
Piulats JM, Espinosa E, De La Merino CL, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol. 2021;39:586–98.
Pham JP, On L, Ardolino L, et al. Efficacy of immune checkpoint inhibition in metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2023;33:316–25.
Moy CS, Albert DM, Diener-West M, et al. Cause-specific mortality coding: methods in the Collaborative Ocular Melanoma Study COMS report no. 14. Control Clin Trials. 2001;22:248–62.
National Comprehensive Cancer Network (NCCN 2022). NCCN clinical practice guidelines in oncology for melanoma: uveal. Version 2.2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488.
Prescribing information for the HEPZATO KIT Hepatic Delivery System. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/201848s000lbl.pdf.
Bethlehem MS, Katsarelias D, Bagge RO. Meta-analysis of isolated hepatic perfusion and percutaneous hepatic perfusion as a treatment for uveal melanoma liver metastases. Cancers Basel. 2021;13:4726.
Bagge RO, Nelson A, Shafazand A, et al. Isolated hepatic perfusion with melphalan for patients with isolated uveal melanoma liver metastases: a multicenter, randomized, open-label, phase III trial (the SCANDIUM trial). J Clin Oncol. 2023;41:3042–50.
Modi S, Gibson T, Vigneswaran G, et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for metastatic uveal melanoma. Melanoma Res. 2022;32:103–11.
Meijer TS, Burgmans MC, de Leede EM, et al. Percutaneous hepatic perfusion with melphalan in patients with unresectable ocular melanoma metastases confined to the liver: a prospective phase II study. Ann Surg Oncol. 2021;28:1130–41.
Burgmans MC, de Leede EM, Martini CH, Kapiteijn E, Vahrmeijer AL, van Erkel AR. Percutaneous isolated hepatic perfusion for the treatment of unresectable liver malignancies. Cardiovasc Intervent Radiol. 2016;39:801–14.
Artzner C, Mossakowski O, Hefferman G, et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: a single center experience. Cancer Imaging. 2019;19:31.
Dewald CLA, Warnke MM, Brüning R, et al. Percutaneous hepatic perfusion (PHP) with melphalan in liver-dominant metastatic uveal melanoma: the German experience. Cancers Basel. 2022;14:118.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Published 28 May 2009 (v4.03: 14 June 2010). U.S. Department of Health and Human Services: National Institutes of Health, National Cancer Institute. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
Edge SB, Byrd DR, Compton CC, et al. (eds). Malignant melanoma of the uvea. In AJCC Cancer Staging Manual. 7th ed. Springer: New York, 2010, pp 547–60.
KIMMTRAK (tebentafusp-tebn) USPI. Immunocore. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761228s000lbl.pdf.
Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014;25:742–6.
Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol. 2015;26:523-32.e2.
de Leede EM, Burgmans MC, Meijer TS, et al. Prospective clinical and pharmacological evaluation of the Delcath system’s second-generation (GEN2) hemofiltration system in patients undergoing percutaneous hepatic perfusion with melphalan. Cardiovasc Intervent Radiol. 2017;40:1196–205.
Tong TML, Samim M, Kapiteijn E, et al. Predictive parameters in patients undergoing percutaneous hepatic perfusion with melphalan for unresectable liver metastases from uveal melanoma: a retrospective pooled analysis. Cardiovasc Intervent Radiol. 2022;45:1304–13.
Meijer TS, Burgmans MC, Fiocco M, et al. Safety of percutaneous hepatic perfusion with melphalan in patients with unresectable liver metastases from ocular melanoma using the Delcath System’s second-generation hemofiltration system: a prospective non-randomized phase II trial. Cardiovasc Intervent Radiol. 2019;42:841–52.
Tong TML, Burgmans MC, Speetjens FM, et al. Combining melphalan percutaneous hepatic perfusion with ipilimumab plus nivolumab in advanced uveal melanoma: first safety and efficacy data from the phase Ib part of the Chopin trial. Cardiovasc Intervent Radiol. 2023;46:350–9.
Acknowledgment
We thank the patients, their families and caregivers, and the study teams at the participating sites. The authors acknowledge Seth Maxwell and Angela Wharton, who are employees of Delcath Systems, Inc., for their involvement in this study, including contributions to data analysis. All the authors critically reviewed the manuscript and approved the final version before submission, including the authorship list. Editorial support was provided by Preetinder Kaur of Certara under the direction of the authors following Good Publication Practice guidelines (Ann Intern Med. 2022;175:1298–1304) and was funded by Delcath Systems Inc., New York, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Jonathan Zager–global principal investigator in the FOCUS phase 3 study and serves on Delcath Systems Medical Advisory Board. Education and Training grant through Delcath. Dr. Marlana Orloff–Advisory Board: Delcath, Replimune. Consultant: Immunocore. Steering Committee: Ideaya. Speaker: Immunocore. Dr. David Eschelman–Received <$5K consulting fees from Delcath. Dr. Evan Glazer–My institution was a site for the clinical trial. I received no direct payment. Delcath paid for the clinical trial to my institution. Dr. J. Howard–I was paid as a consultant to Delcath as a proctor for this trial to ensure new institutions adhered to protocol. Prof. Erika Richtig–Medical University of Graz received payments for conducting the Phase 3 study. Dr. Sebastian Ochsenreither–Advice: Immunocore, Delcath, Janssen. Speakers’ bureau: Immunocore. Dr. Georgia Beasley–Clinical trial funding paid to institution from Replimune, checkmate pharmaceuticals, Philogen, Delcath. Advisory board BMS 10.2023. Dr. Anja Gesierich–University Hospital Würzburg received payments for conducting the Phase 3 study. Professor Reinhard Dummer–intermittent, project focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer, Simcere and touchIME outside the submitted work. Dr. Ana Arance–Consulting or advisory role: Pierre- Fabre, Novartis, Roche, BMS, MSD, Biontech. Speakers’ Bureau: Pierre- Fabre, Novartis, Roche, BMS, MSD. Travel, Accommodations, Expenses: BMS, MSD. Mr. Stephen Fenwick–Liverpool University Hospitals NHS Trust received support for conducting this Phase 3 study from the trial sponsor. Dr. Joseph Sacco–I have been a paid member of advisory boards for Delcath, Replimune and Immunocore. I have received grant funding from Astra Zeneca and BMS (paid to institution). I have had travel and conference attendance paid for by MSD, BMS and Replimune. Dr. Matthew Wheater–Institutional (University Hospital Southampton) funding for conducting a commercial trial. Personal funding for participation in advisory board for Delcath.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zager, J.S., Orloff, M., Ferrucci, P.F. et al. Efficacy and Safety of the Melphalan/Hepatic Delivery System in Patients with Unresectable Metastatic Uveal Melanoma: Results from an Open-Label, Single-Arm, Multicenter Phase 3 Study. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15293-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15293-x