Abstract
Background
Upper gastrointestinal cancers are aggressive malignancies with poor prognosis, even following multimodality therapy. As such, they require timely and accurate diagnostic and surveillance strategies; however, such radiological workflows necessitate considerable expertise and resource to maintain. In order to lessen the workload upon already stretched health systems, there has been increasing focus on the development and use of artificial intelligence (AI)-centred diagnostic systems. This systematic review summarizes the clinical applicability and diagnostic performance of AI-centred systems in the diagnosis and surveillance of esophagogastric cancers.
Methods
A systematic review was performed using the MEDLINE, EMBASE, Cochrane Review, and Scopus databases. Articles on the use of AI and radiomics for the diagnosis and surveillance of patients with esophageal cancer were evaluated, and quality assessment of studies was performed using the QUADAS-2 tool. A meta-analysis was performed to assess the diagnostic accuracy of sequencing methodologies.
Results
Thirty-six studies that described the use of AI were included in the qualitative synthesis and six studies involving 1352 patients were included in the quantitative analysis. Of these six studies, four studies assessed the utility of AI in gastric cancer diagnosis, one study assessed its utility for diagnosing esophageal cancer, and one study assessed its utility for surveillance. The pooled sensitivity and specificity were 73.4% (64.6–80.7) and 89.7% (82.7–94.1), respectively.
Conclusions
AI systems have shown promise in diagnosing and monitoring esophageal and gastric cancer, particularly when combined with existing diagnostic methods. Further work is needed to further develop systems of greater accuracy and greater consideration of the clinical workflows that they aim to integrate within.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal cancer is an aggressive cancer with a mean estimated 5-year survival rate of 35–45%, even after treatment with curative intent.1, 2 The reported survival rate in advanced-stage disease drops further to 5–10% and can be attributed to the malignancy’s insidious onset and aggressive tumor biology that often favors recurrence.3,4,5 Similarly, gastric cancer has a poor 5-year survival rate and is still the third leading cause of malignancy-related death worldwide.6 A number of investigations, such as computed tomography (CT) scans, positron emission tomography (PET) scans, endoscopic ultrasound (EUS), and endobronchial ultrasound (EBUS), are utilized in the diagnostic and staging pathway of esophagogastric (EG) malignancy, with CT being the most commonly used of those that are noted.7 Unlike colorectal, hepatocellular, and pancreatic cancers, there is no reliable biomarker that can be tested and tracked non-invasively for diagnostic or surveillance purposes in esophageal and gastric cancers.8,9,10 Consequently, patients are often reliant on radiological investigations for diagnosis with staging, detection of recurrence, and monitoring response to treatment.7 These workflows necessitate both timely and expert radiological interpretation, a requirement that is often difficult to achieve given busy clinical work schedules and a lack of expertise outside tertiary oncological centers. As such, there has been increasing calls to explore the use of AI-centred diagnostic systems to alleviate this issue.
In the context of medical diagnostics, AI is the use of a system to mimic human cognition in the comprehension, analysis, and presentation of medical data.11,12,13 This is often achieved using machine learning (ML), which is a specialized sub-field within AI that improves the performance of systems through repetitive experience. For example, in EG cancers, ML has been used extensively by AI systems to understand endoscopy images and enhance the interpretation of solely operator-dependent endoscopy.14,15,16 Naturally, the next step will be the integration of AI into the major imaging modalities used in the management of EG cancers, specifically CT scans. Typically, this involves the high-throughput extraction of large quantities of data from the images and is a technique termed as radiomics. Radiomics is an emerging field using a non-invasive approach to extract numerous quantitative features from medical images, especially parameters not visible to the naked human eye or quantifiable by routine analysis.17, 18 Specifically, with CT scans, radiomics offers the unique advantage of combining ML to acquire images; segment images into regions of interest (ROIs) or volumes of interest (VOIs); extraction of quantitative imaging features from ROIs and VOIs; and, lastly, constructing and validating models. Recently, there has been an increase in work reporting on the combined or individual use of AI or radiomics to diagnose or monitor EG cancers. This review aims to summarize the potential applicability of AI diagnostic systems in the diagnosis and surveillance of esophageal and gastric cancers.
Methods
Literature search methods, inclusion and exclusion criteria, outcome measures, and statistical analysis were defined according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 Patients were not involved in the conception, design, analysis, drafting, interpretation, or revision of this research, hence ethical approval was not required and was thus not sought for this study.
Literature Search
The following databases were searched: MEDLINE (from 1946 until the first week of April 2021) via OvidSP; MEDLINE In-Process and other non-indexed citations (latest issue) via OvidSP; Ovid EMBASE (from 1974 to the latest issue); and Scopus (from 1996 until the present). The last search was performed on 15 April 2021. Search terms used several strings that were linked by standard modifiers in the following order: ‘machine learning’, ‘artificial intelligence’, ‘radiomic’, ‘AI’ OR ‘ML’, as well as ‘esophageal cancer’, ‘esophageal squamous cell cancer’, ‘esophageal adenocarcinoma’, ‘ESCC’, ‘EAC’, ‘esophageal malignancy’, ‘upper gastrointestinal cancer’, OR ‘upper GI cancer’. Additionally, the references of included articles were hand-searched to identify any additional studies.
Selection and Quality Assessment of Studies
Articles were screened for eligibility by SC and VS, and, where conflict arose, a third co-author (SRM) was consulted. Studies were included if they had incorporated the use of AI-centred systems in CT imaging for evaluating both esophageal and gastric cancers. Studies with diagnostic, prognostic, and monitoring intents were included. Studies were excluded if they did not evaluate ML, used imaging modalities other than CT, did not include patients with esophageal or gastric cancers, had incomplete data on outcome measures, were not written in the English language, had sample sizes fewer than 30 patients, or had incompatible designs, including letters, comments and reviews. Studies were assessed for robustness of methodology using the Quality Assessment Tool for Diagnostic Accuracy Studies 2 (QUADAS-2), which comprises four domains covering patient selection, index test, reference standard, and flow of patients through the study and timing of the index test(s) and reference standard. Each domain is evaluated in terms of the risk of bias, and the first three domains are also assessed for any concerns regarding applicability. In doing so, this highlights aspects of the study design that may be exposed to bias.
Statistical Analysis
All statistical analyses were performed using STATA/SE version 16.0 (StataCorp LLC, College Station, TX, USA). The overall pooled estimate of sensitivity and specificity, with their corresponding 95% confidence intervals (CIs), was calculated using the random-effects model with the metandi command in STATA/SE. Sensitivity was defined as the proportion of patients with esophageal cancer who were correctly confirmed by AI, while specificity was defined as correctly identifying patients without the disease. Forest plots were used to visualize the variation of the diagnostic parameter effect size estimates with 95% CI and weights from the included studies.
Results
Study Selection
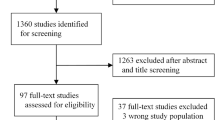
The database search yielded a total of 1439 studies, of which 137 duplicates were removed. Titles and abstracts of the remaining 1302 studies were screened for eligibility and 648 studies were removed. A further 617 studies were excluded after full-text review due to incompatible outcome measures, study design, or small sample sizes of fewer than 30 patients (Fig. 1). Thirty-seven studies that described the use of ML (a branch of AI) platforms for the diagnosis and surveillance of esophageal and gastric cancers were included in this study (Table 1).
Quality Appraisal
Assessment of studies using the QUADAS-2 tool showed a low level of bias among the studies (Table 2). The risk of bias and concerns on their applicability was low across most domains. Some risk of bias was present due to the heterogeneity of the patients included; however, in most studies, there was little reporting of the sensitivity and specificity of the ML algorithms used.
Use of Machine Learning and Radiomics in the Management of Esophageal Cancer
Takeuchi et al. reported diagnostic accuracy of 84% (sensitivity 71.7%; specificity 90.0%) in detecting stage T1–T5 esophageal cancer in 46 patients.20 One study looked at the prognosis of patients with esophageal cancer, in which Foley et al. reported six variables to be predictive of overall survival in their work of 405 patients.21 Two studies evaluated the use of ML to assess response to chemoradiotherapy for esophageal cancers. The model developed by Wang et al. to evaluate the scan of 131 patients who underwent neoadjuvant chemotherapy diagnosed lymph node metastasis better than the preoperative short axis size of the largest lymph node on CT, with an area under the curve (AUC) of 0.887.22 Jin et al. combined a radiomics and dosimetric approach and reported an AUC of 0.708 in predicting the treatment response of patients with esophageal cancer who underwent chemoradiotherapy.23
Use of Machine Learning and Radiomics in the Management of Gastric Cancer
Two studies investigated the use of radiomics in diagnosing gastric cancer, specifically in differentiating gastric cancer from other gastric lesions.24, 25 In their study evaluating VOI-based textural features on preoperative arterial phase and portal phase scans of 95 patients, Ba-Ssalamach et al. differentiated gastric adenocarcinoma with an error rate as low as 3.1%.24 Two studies reported that there was little correlation between radiomic features and histological grades, with AUCs below 0.7,9, 10 while five studies evaluated images for lymph node status, vascular invasion, and occult peritoneal metastasis, with AUCs as high as 0.941.11,12,13,14,15 Of the included studies, two studies evaluated the use of AI for prognosis after surgical resection for gastric cancers. Li et al. extracted 273 features from each ROI and 485 features from each VOI, and used the least absolute shrinkage and selection operator (LASSO) method to predict overall survival, although the results were not promising in their test set.26 In contrast, Giganti et al. extracted 107 features from each VOI that were significantly associated with a negative overall survival in patients with resectable gastric cancer.27 Four studies also investigated the use of AI for predicting response to neoadjuvant chemotherapy. Giganti et al. determined 14 features in pretreatment arterial phase images that were significantly different between responders and non-responders, while another study by Li et al. showed similar results with portal venous phase images.28, 29 In their multicenter study, Jiang et al. identified potential predictors from portal venous phase scans of 1591 patients that were significantly different between responders and non-responders to neoadjuvant chemotherapy and predictive of disease-free survival.30, 31 Two studies evaluated the response to targeted immunotherapy with trastuzumab or radiotherapy. Hou et al. showed that radiomic signatures can predict response to radiotherapy with an AUC of 0.749, while Yoon et al. reported AUCs of 0.75–0.77 in their small pilot study of 26 cases of HER2-positive gastric cancer treated with trastuzumab.32, 33
Artificial Intelligence as a Diagnostic and Monitoring Tool: Quantitative Analysis
Six studies involving 1352 patients provided sufficient data of true positive, true negative, false positive, and false negative rates for the calculation of sensitivity and specificity. Of these studies, four studies assessed its utility in gastric cancer diagnosis, one study assessed its utility for diagnosing esophageal cancer, and one study assessed its utility for surveillance (Table 1). The pooled sensitivity and specificity were 73.4% (64.6–80.7) and 89.7% (82.7–94.1), respectively, as visualized on the forest plot and summary receiver operating characteristic curve (Figs. 2 and 3).
Discussion
Our systematic review shows that the application of radiomics and AI for the diagnosis and surveillance of upper gastrointestinal tract malignancies is promising, despite being in its nascency. The included radiological studies show that AI can be potentially used to diagnose cancers, differentiate malignancies from benign lesions, and detect occult disease. AI systems may also be used for staging disease, determining if surgery will improve survival outcomes in patients with resectable disease, and in predicting whether patients will respond to adjuvant or neoadjuvant chemoradiotherapy. Our paper also highlights the different AI platforms available for these purposes and captures their breadth.
The typical patient undergoes several CT scans during their journey, with diagnosis as the primary aim. Combining radiomics and AI to current scans will enable clinicians to simultaneously predict how they will respond to treatment and also assess how they have responded to treatment. In other cancers, radiomic data have provided support to genomic data in generating a prognostic signature that exceeds the accuracy of traditional TNM staging.34 Given that there is a direct correlation between histopathological response of patients who underwent chemoradiotherapy and the overall survival rate, the ability to assess clinical response will be useful in adjusting the dose and regimes of chemoradiotherapy.35, 36 Our paper has included at least one study using radiomics or AI to assess the response to surgery, chemotherapy, radiotherapy and immunotherapy, and all report high performance; however, there is still scarce evidence to add support to existing studies described here.
AI can also help in overcoming any technical limitations faced by traditional imaging. For example, Jin et al. combined radiomic and dosimetric analyses to overcome the artefacts in wall thickness created by the regular peristaltic waves of contraction.23 In another study, Ding et al. showed that their models detected occult peritoneal metastasis more accurately than conventional CT scans. Previous studies including the Worldwide Esophageal Cancer Collaboration have reported that survival decreases with the presence of lymph node metastases, and imaging examinations are often the first-line investigations for assessing most lymph node statuses in esophageal cancer.37,38,39 However, the accuracy of CT in diagnosing the N stage of esophageal cancer was just 59%.40 Most clinicians use a size criterion of 1 cm to differentiate between benign and malignant enlargement of lymph nodes but this only has a sensitivity of 30–60% and a somewhat higher specificity of 60–80%.41,42,43 In their study, Wang et al. showed that support vector machine (SVM) models have better diagnostic capability for lymph node metastasis than the traditional LN size criteria.22 Furthermore, Bollschweiler et al. used a different ML methodology, termed artificial neural network (ANN), and reported a diagnostic accuracy of 79% in predicting LN metastasis in esophageal cancer.44
Strength and Limitations
The strength of our systematic review lies in its up-to-date unified analysis of esophageal and gastric cancers in different countries. We also identified challenges that will need to be overcome for the technology to be implemented into daily clinical practice. Our study has several weaknesses. First, most of the articles included in the study did not report the specificity or sensitivity of their AI technologies, which prevented a more comprehensive quantitative analysis to achieve a pooled statistic for the diagnostic accuracy of AI. This also prevented the stratification of pooled data based on study intent (diagnostic vs. prognostic). Furthermore, the diagnostic or predictive accuracy of AI depends on several parameters, including the specific AI program or model developed, scanning equipment, image preprocessing, acquisition protocols, and image reconstruction algorithms.
Although there is heterogeneity between the studies, most of the work is limited to a few specific groups that have taken an interest in this field. The majority of the studies are based in Asia, and several of the included papers stem from the work of the same group. Hence, within the same group, the data acquisition and processing techniques are identical but the aims of the study were different and hence merited inclusion. For example, in the studies by Jiang et al., the first study evaluated the use of radiomics and AI in characterizing the tumor microenvironment, while the other study focused on identifying occult metastasis.30, 31 Another example are the smaller studies by Giganti et al., each of which separately investigate the response to curative resection and chemotherapy.27, 28 Together, these studies shed light on a different aspect of the tumor biology of gastric cancers. In the same vein, we also included some studies with a sample size that was <100. Although small sample sizes lend to a greater degree of variation on the quantitative analysis, these studies were relevant in studying a niche area of treatment response. Larger studies have previously tended to focus on the diagnostic aspects, while other facets such as monitoring for recurrence, response to curative resection and chemotherapy, and tumor heterogeneity are areas that are still in their infancy and hence studied at a smaller level. Furthermore, this emphasizes the paucity of studies of large sample sizes and hints at areas that need further work within the field of AI in esophagogastric cancers.
Future Directions
Future work should be aimed at the ‘in silico’ bench to bedside translation of these technologies. Although we highlight much promise in these technologies, several factors require evaluation prior to these technologies being employed in routine upper gastrointestinal oncological care:
-
(1)
Use case: There needs to be early clarification in the lifecycle of these AI devices as to (1) their specific clinical task; (2) potential risk and benefits; (3) whether they are used within either new or existing clinical workflows; and (4) whether they are used independently to diagnose disease/recurrence or as a ‘second reader’ alongside a human clinician. Downstream validation of these systems is dictated by many of these early decisions.
-
(2)
Model development: The development of these systems are reliant on diverse, large-scale, and well-maintained datasets that are accurately labeled for the purposes of model training and internal validation. Systems created upon small single-center datasets with post hoc labeling rarely perform well when subjected to out-of-set testing.
-
(3)
Validation: Independent validation of AI systems is crucial, with comparison against expert clinicians to demonstrate either non-inferiority or superiority in diagnostic performance to be undertaken when feasible. Such evaluations require careful study planning, with the need for diverse demographic representation in test datasets in order to assess for bias.
-
(4)
Infrastructural requirements: Aside from developer considerations, the bottleneck for many contemporary AI products is the end-user adoption and experience. There needs to be careful consideration of the IT infrastructural requirements at hospitals in which these technologies may be reasonably deployed.
-
(5)
Cost effectiveness: Lastly, although it is assumed that the introduction of AI systems will lead to cost saving across health systems, this requires formal quantification. If deemed not to be financially beneficial, it may be more cost effective to hire diagnostic clinicians, which is the focus on current large-scale studies.
Furthermore, the power of these models is dependent on a large and diverse diet of datasets. At present, the retrospective single-center work available is insufficient and is limited in size, scope and variety. Given that the largest advances in esophagogastric surgery have occurred based on large prospective studies, the advent of ML only calls for further collaborative efforts at an international level to fully reap the potential of this technology.
Conclusion
AI and radiomics have a huge potential for diagnostic and surveillance of esophageal and gastric cancers. There is currently a paucity of large-scale studies evaluating the usefulness of AI and radiomics in esophageal cancer and the evidence is limited to retrospective studies of small sample sizes. Further progression of its clinical application will require collaborative efforts to generate a large and diverse dataset that can produce an accurate model. This relies on determining the best and most feasible methodology for ML and standardizing this across centers. Hence, further work should focus on these areas.
References
Rouvelas I, Zeng W, Lindblad M, Viklund P, Ye W, Lagergren J. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol. 2005;6(11):864–70.
Davies AR, Pillai A, Sinha P, Sandhu H, Adeniran A, Mattsson F, et al. Factors associated with early recurrence and death after esophagectomy for cancer. J Surg Oncol. 2014;109(5):459–64.
Li Y, Qin J, Li X, Fu X, Liu L, Liu Y, et al. Investigation to metastasis of regional lymph node station and prediction to long-term survival following esophagectomy in thoracic esophageal cancer with stage T1 to T3. J Clin Oncol. 2019;37(15 Suppl):e15519–e15519.
Besharat S, Jabbari A, Semnani S, Keshtkar A, Marjani J. Inoperable esophageal cancer and outcome of palliative care. World J Gastroenterol. 2008;14(23):3725–8.
Zhu ZJ, Hu Y, Zhao YF, Chen XZ, Chen LQ, Chen YT. Early recurrence and death after esophagectomy in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91(5):1502–8.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–72.
Battaglin F, Naseem M, Puccini A, Lenz HJ. Molecular biomarkers in gastro-esophageal cancer: recent developments, current trends and future directions. Cancer Cell Int. 2018;18(1):99.
Westerterp M, Van Westreenen HL, Reitsma JB, Hoekstra OS, Stoker J, Fockens P, et al. Esophageal cancer: CT, endoscopie US, and FDG PET for assessment of response to neoadjuvant therapy-systematic review. Radiology. 2005;236(3):841–51.
Tan C, Qian X, Guan Z, Yang B, Ge Y, Wang F, et al. Potential biomarkers for esophageal cancer. SpringerPlus. 2016;5:467.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–6.
Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imag. 2012;30(9):1234–48.
McCarthy J, Minsky ML, Rochester N, Shannon CE. A proposal for the Dartmouth summer research project on artificial intelligence. AI Mag. 2006;27(4).
Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2(1):35.
Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. npj Digit Med. 2020;3(1):118.
Niu PH, Zhao LL, Wu HL, Zhao DB, Chen YT. Artificial intelligence in gastric cancer: application and future perspectives. World J Gastroenterol. 2020;26(36):5408–19.
Kailin J, Xiaotao J, Jinglin P, Yi W, Yuanchen H, Senhui W, et al. Current evidence and future perspective of accuracy of artificial intelligence application for early gastric cancer diagnosis with endoscopy: a systematic and meta-analysis. Front Med. 2021;8:629080.
Hirasawa T, Ikenoyama Y, Ishioka M, Namikawa K, Horiuchi Y, Nakashima H, et al. Current status and future perspective of artificial intelligence applications in endoscopic diagnosis and management of gastric cancer. Digest Endosc. 2021;33(2):263–72.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. 2021;372:n71.
Takeuchi M, Seto T, Hashimoto M, Ichihara N, Morimoto Y, Kawakubo H, et al. Performance of a deep learning-based identification system for esophageal cancer from CT images. Esophagus. 2021;18(3):612–20.
Foley KG, Hills RK, Berthon B, Marshall C, Parkinson C, Lewis WG, et al. Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol. 2018;28(1):428–36.
Wang ZL, Zhou ZG, Chen Y, Li XT, Sun YS. Support vector machines model of computed tomography for assessing lymph node metastasis in esophageal cancer with neoadjuvant chemotherapy. J Comput Assist Tomogr. 2017;41(3):455–60.
Jin X, Zheng X, Chen D, Jin J, Zhu G, Deng X, et al. Prediction of response after chemoradiation for esophageal cancer using a combination of dosimetry and CT radiomics. Eur Radiol. 2019;29(11):6080–8.
Ba-Ssalamah A, Muin D, Schernthaner R, Kulinna-Cosentini C, Bastati N, Stift J, et al. Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol. 2013;82(10):e537–43.
Ma Z, Fang M, Huang Y, He L, Chen X, Liang C, et al. CT-based radiomics signature for differentiating Borrmann type IV gastric cancer from primary gastric lymphoma. Eur J Radiol. 2017;91:142–7.
Li W, Zhang L, Tian C, Song H, Fang M, Hu C, et al. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur Radiol. 2019;29(6):3079–89.
Giganti F, Antunes S, Salerno A, Ambrosi A, Marra P, Nicoletti R, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27(5):1831–9.
Giganti F, Marra P, Ambrosi A, Salerno A, Antunes S, Chiari D, et al. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: comparison with tumour regression grade at final histology. Eur J Radiol. 2017;90:129–37.
Li Z, Zhang D, Dai Y, Dong J, Wu L, Li Y, et al. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: a pilot study. Chinese J Cancer Res. 2018;30(4):406–14.
Jiang Y, Yuan Q, Lv W, Xi S, Huang W, Sun Z, et al. Radiomic signature of 18F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics. 2018;8(21):5915–28.
Jiang Y, Chen C, Xie J, Wang W, Zha X, Lv W, et al. Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine. 2018;36:171–82.
Hou Z, Yang Y, Li S, Yan J, Ren W, Liu J, et al. Radiomic analysis using contrast-enhanced CT: predict treatment response to pulsed low dose rate radiotherapy in gastric carcinoma with abdominal cavity metastasis. Quant Imag Med Surg. 2018;8(4):410–20.
Yoon SH, Kim YH, Lee YJ, Park J, Kim JW, Lee HS, et al. Tumor heterogeneity in human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer assessed by CT texture analysis: association with survival after trastuzumab treatment. PLoS One. 2016;1(8):e0161278.
Van Rossum PSN, Fried DV, Zhang L, Hofstetter WL, Van Vulpen M, Meijer GJ, et al. The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med. 2016;57(5):691–700.
Chao YK, Chan SC, Liu YH, Chen HW, Wan YL, Chang HK, et al. Pretreatment T3–4 stage is an adverse prognostic factor in patients with esophageal squamous cell carcinoma who achieve pathological complete response following preoperative chemoradiotherapy. Ann Surg. 2009;249(3):392–6.
Tong DKH, Law S, Kwong DLW, Chan KW, Lam AKY, Wong KH. Histological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factor. Ann Surg Oncol. 2010;17(8):2184–92.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Ajani JA, et al. Treatment outcomes of resected esophageal cancer. Annal Surg. 2002;236(3):376–84.
Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–22.
Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol. 2002;41(2):161–7.
Picus D, Balfe DM, Koehler RE, Roper CL, Owen JW. Computed tomography in the staging of esophageal carcinoma. Radiology. 1983;146(2):433–8.
Dorfman RE, Alpern MB, Gross BH, Sandier MA. Upper abdominal lymph nodes: Criteria for normal size determined with CT. Radiology. 1991;180(2):319–22.
Yokota T, Igaki H, Kato K, Tsubosa Y, Mizusawa J, Katayama H, et al. Accuracy of preoperative diagnosis of lymph node metastasis for thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin Oncol. 2016;21(2):283–8.
Bollschweiler EH, Mönig SP, Hensler K, Baldus SE, Maruyama K, Hölscher AH. Artificial neural network for prediction of lymph node metastases in gastric cancer: a phase II diagnostic study. Ann Surg Oncol. 2004;11(5):506–11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chidambaram, S., Sounderajah, V., Maynard, N. et al. Diagnostic Performance of Artificial Intelligence-Centred Systems in the Diagnosis and Postoperative Surveillance of Upper Gastrointestinal Malignancies Using Computed Tomography Imaging: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Ann Surg Oncol 29, 1977–1990 (2022). https://doi.org/10.1245/s10434-021-10882-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10882-6