Abstract
Background
Reoperation rates following breast-conserving surgery (BCS) range from 10 to 40%, with marked surgeon and institutional variation.
Objective
The aim of this study was to identify factors associated with intraoperative margin re-excision, evaluate for any differences in local recurrence based on margin re-excision and determine reoperation rates with use of intraoperative margin analysis.
Patients and Methods
We analyzed consecutive patients with ductal carcinoma in situ (DCIS) or invasive breast cancer who underwent BCS at our institution between 1 January 2005 and 31 December 2016. Routine intraoperative frozen section margin analysis was performed and positive or close margins were re-excised intraoperatively. Univariate analysis was used to compare margin status and the Kaplan–Meier method was used to compare recurrence. Multivariable logistic regression was utilized to analyze factors associated with re-excision.
Results
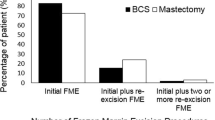
We identified 3201 patients who underwent BCS—688 for DCIS and 2513 for invasive carcinoma. Overall, 1513 (60.2%) patients with invasive cancer and 434 (63.1%) patients with DCIS had close or positive margins that underwent intraoperative re-excision. Margin re-excision was associated with larger tumor size in both groups. The permanent pathology positive margin rate among all patients was 1.2%, and the 30-day reoperation rate for positive margins was 1.1%. Five-year local recurrence rates were 0.6% and 1.2% for patients with DCIS and invasive cancer, respectively. There was no difference in recurrence between patients with and without intraoperative margin re-excision (p = 0.92).
Conclusion
Both DCIS and invasive carcinoma had similar rates of intraoperative margin re-excision. Although intraoperative margin re-excision was common, the reoperation rate was extremely low and there was no difference in recurrence between those with or without intraoperative re-excision.
Similar content being viewed by others
References
Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: a meta-analysis of individual patient data for 10,801 women in 17 randomized trials. Lancet. 2011; 378(9804):1707–16.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347(16):1233–41.
Bernardi S, Bertozzi S, Londero AP, Gentile G, Angione V, Petri R. Influence of surgical margins on the outcome of breast cancer patients: a retrospective analysis. World J Surg. 2014; 38(9):2279–87.
Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014; 21(3):717–30.
O’Kelly Priddy CM, Forte VA, Lang JE. The importance of surgical margins in breast cancer. J Surg Oncol. 2016; 113(3):256–63.
McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012; 307:467–75.
Singh M, Singh G, Hogan KT, Atkins KA, Schroen AT. The effect of intraoperative specimen inking on lumpectomy re-excision rates. World J Surg Oncol. 2010; 8:4.
Margenthaler JA, Gao F, Klimberg VS. Margin index: a new method for prediction of residual disease after breast-conserving surgery. Ann Surg Oncol. 2010; 17:2696–701.
Balch GC, Mithani SK, Simpson JF, Kelley MC. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. Am Surg. 2005; 71(1):22–7; discussion 27–8.
Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery (SM) database. Ann Surg Oncol. 2014; 21(10):3185–91.
Rivere AE, Chiasson KF, Corsetti RL, Fuhrman GM. An assessment of margins after lumpectomy in breast cancer management. Am Surg. 2016; 82(2):156–60.
Sheikh F, Pockaj B, Wasif N, Dueck A, Gray RJ. Positive Margins After breast-conserving therapy: localization technique or tumor biology. Am J Surg. 2011; 202(3):281–5.
Jorns JM, Visscher D, Sabel M, et al. Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases re-operative rates: one-year experience at an Ambulatory Surgical Center. Am J Clin Pathol. 2012; 138(5):657–69.
Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007; 14(4):1458–71.
Camp ER, McAulifee PG, Gilroy JS, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005; 201(6):855–61.
Chagpar A, Yen T, Sahin AA, et al. Intraoperative margin assessment reduces reexcision rates in patients with ductal carcinoma in situ treated with breast-conserving surgery. Am J Surg. 2003; 186(4):371–7.
Fukamachi K, Ishida T, Usami S, et al. Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol. 2010; 40(6):513–20.
Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the national surgical quality improvement program data. Surgery. 2014; 156(1):190–7.
Osborn JB, Keeney GL, Jakub JW, Degnim AC, Boughey JC. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol. 2011; 18(11):3204–9.
Wilson LB. A method for the rapid preparation of fresh tissues for the microscope. JAMA. 1905; 45:1737.
Ferreiro JA, Myers JL, Bostwick DG. Accuracy of frozen section diagnosis in surgical pathology: review of a 1-year experience with 24,880 cases at Mayo Clinic Rochester. Mayo Clin Proc. 1995; 70(12):1137–41.
Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014; 21(3):704–16.
Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016; 23(12):3801–10.
Agostinho JL, Zhao X, Sun W, et al. Prediction of positive margins following breast conserving surgery. Breast. 2015; 24(1):46–50.
Barentsz MW, Potsma EL, van Dalen T, et al. Prediction of positive resection margins in patients with non-palpable breast cancer. Eur J Surg Oncol. 2015; 41(1):106–12.
Jia H, Jia W, Yang Y, et al. HER-2 positive breast cancer is associated with an increased risk of positive cavity margins after initial lumpectomy. World J Surg Oncol. 2014; 12:289.
Hughes JH, Mason MC, Gray RJ, et al. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J. 2008; 14(2):153–7.
Jeevan R, Cromwell DA, Trivella M, et al. Reoperation rates after breast conserving surgery for breast cancer among women in england: retrospective study of hospital episode statistics. BMJ. 2012; 345:e4505.
Talsma AK, Reedjik AM, Damhuis RA, Westenend PJ, Vles WJ. Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch Hospitals. Eur J Surg Oncol. 2011; 37(4):357–63.
Murphy BL, Boughey JC, Keeney MG, et al. Factors associated with positive margins in women undergoing breast conservation surgery. Mayo Clin Proc. 2018; 93(4):429–35.
Jorns JM, Daignault S, Sabel MS, Myers JL, Wu AJ. Frozen sections in patients undergoing breast conserving surgery at a single ambulatory surgical center: 5 year experience. Eur J Surg Oncol. 2017; 43(7):1273–81.
Vos EL, Gaal J, Verhoef C, Brouwer K, van Deurzen CHM, Koppert LB. Focally positive margins in breast conserving surgery: predictors, residual disease, and local recurrence. Eur J Surg Oncol. 2017; 43(10):1846–54.
Aziz D, Rawlison E, Narod SA, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006; 12(4):331–7.
O’Sullivan MJ, Li T, Freedman G, Morrow M. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007; 14(11):3133–40.
Kouzminova NB, Aggarwal S, Aggarwal A, Allo MD, Lin AY. Impact of initial surgical margins and residual cancer upon re-excision on outcome of patients with localized breast cancer. Am J Surg. 2009; 198(6):771–80.
Ali AN, Vapiwala N, Guo M, Hwang WT, Harris EE, Solin LJ. The impact of re-excision and residual disease on local recurrence after breast conservation treatment for patients with early stage breast cancer. Clin Breast Cancer. 2011; 11(6):400–5.
Gray RJ, Salud C, Nguyen K, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol. 2001; 8(9):711–5.
Jakub JW, Gray RJ, Degnim AC, Boughey JC, Gardner M, Cox CE. Current status of radioactive seed for localization of non-palpable breast lesions. Am J Surg. 2010; 199(4):522–8.
Murphy JO, Moo TA, Kina TA, et al. Radioactive seed localization compared to wire localization in breast-conserving surgery: initial 6-month experience. Ann Surg Oncol. 2013; 20(13):4121–7.
Sharek D, Zuley ML, Zhang JY, Soran A, Ahrendt GM, Ganott MA. Radioactive seed localization versus wire localization for lumpectomies: a comparison of outcomes. AJR Am J Roentgenol. 2015; 204(4):872–7.
Langhans L, Tvedskov TF, Klausen TL, et al. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized multicenter, open-label trial. Ann Surg. 2017; 266(1):29–35.
Bloomquist EV, Ajkay N, Patil S, Collett AE, Frazier TG, Barrio AV. A randomized prospective comparison of patient-assessed satisfaction and clinical outcomes with radioactive seed localization versus wire localization. Breast J. 2016; 22(2):151–7.
Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol. 2011; 18(12):3407–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jennifer M. Racz, Amy E. Glasgow, Gary L. Keeney, Amy C. Degnim, Tina J. Hieken, James W. Jakub, John C. Cheville, Elizabeth B. Habermann, and Judy C. Boughey have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Racz, J.M., Glasgow, A.E., Keeney, G.L. et al. Intraoperative Pathologic Margin Analysis and Re-Excision to Minimize Reoperation for Patients Undergoing Breast-Conserving Surgery. Ann Surg Oncol 27, 5303–5311 (2020). https://doi.org/10.1245/s10434-020-08785-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08785-z