Abstract
Background
Rising use of multigene panel testing has led to increased identification of variants of uncertain significance (VUS). Consensus guidelines state that clinicians should not make medical management decisions based on VUS findings. We sought to analyze how VUS affect management of patients at risk for hereditary breast cancer.
Methods
All genetic testing reports for indications of hereditary breast cancer risk from a single tertiary-care institution from 2015 to 2018 were reviewed. Variants were grouped by pathogenicity (benign/likely benign, VUS, or pathogenic/likely pathogenic [P/LP]) and by breast cancer susceptibility (high, moderate, or none). Patient and management characteristics were compared by variant pathogenicity and breast cancer risk.
Results
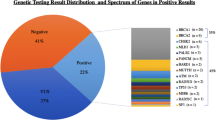
Overall, 563 patients underwent genetic testing for breast cancer risk; 336 VUS were identified in 228 (40.5%) of patients of which 26.4% were in high or moderate penetrance genes. P/LP results were found in 61 (10.8%) patients, of which 61.2% were identified in breast-specific moderate and high penetrance genes, and 38.7% were found in non-breast specific genes. Of variants found in high-risk genes, 54.5% were P/LP and 45.5% were VUS. On multivariable analysis, prophylactic mastectomy was associated with younger age and personal history of cancer, but not variant pathogenicity or penetrance. There were no differences in the use of post-test imaging, oophorectomy, or colonoscopy based on variant findings or age.
Conclusions
In this era of multigene panel testing, genetic factors help to inform, but not dictate, complex decision-making in surveillance and management of patients at risk for hereditary breast cancer.

Similar content being viewed by others
References
Hall J, Lee M, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–9.
Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–90.
Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57.
O’Leary E, Iacoboni D, Holle J, et al. Expanded gene panel use for women with breast cancer: identification and intervention beyond breast cancer risk. Ann Surg Oncol. 2017;24:3060–6.
Yang S, Axilbund JE, O’Leary E, et al. Underdiagnosis of hereditary breast and ovarian cancer in medicare patients: genetic testing criteria miss the mark. Ann Surg Oncol. 2018;25:2925–31.
Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453–60.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4:1066–72.
Eccles BK, Copson E, Maishman T, Abraham JE, Eccles DM. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15:936.
Scherr CL, Lindor NM, Malo TL, Couch FJ, Vadaparampil ST. Genetic counselors’ practices and confidence regarding variant of uncertain significance results and reclassification from BRCA testing. Clin Gen. 2015;88:523–9.
Eccles DM, Mitchell G, Monteiro AN, et al. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol. 2015;26:2057–65.
Genetic/Familial High-Risk Assessment: Breast and ovarian. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed 21 Apr 2019.
Howarth DR, Lum SS, Esquivel P, Garberoglio CA, Senthil M, Solomon NL. Initial results of multigene panel testing for hereditary breast and ovarian cancer and lynch syndrome. Am Surg. 2015;81:941–4.
Milliron KJ, Griggs JJ. Advances in genetic testing in patients with breast cancer, high-quality decision making, and responsible resource allocation. J Clin Oncol. 2019;37:445–7.
Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35:2232–9.
Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24:3067–72.
Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226:560–5.
Henry DA, Lee MC, Almanza D, et al. Trends in use of bilateral prophylactic mastectomy vs high-risk surveillance in unaffected carriers of inherited breast cancer syndromes in the Inherited Cancer Registry (ICARE). Breast Cancer Res Treat. 2019;174:39–45.
Gilbert E, Zabor EC, Stempel M, Mangino D, Heerdt A, Pilewskie M. Differences among a modern cohort of BRCA mutation carriers choosing bilateral prophylactic mastectomies compared to breast surveillance. Ann Surg Oncol. 2017;24:3048–54.
Boughey JC, Attai DJ, Chen SL, et al. Contralateral prophylactic mastectomy consensus statement from the American Society of Breast Surgeons: additional considerations and a framework for shared decision making. Ann Surg Oncol. 2016;23:3106–11.
Hunt KK, Euhus DM, Boughey JC, et al. Society of surgical oncology breast disease working group statement on prophylactic (risk-reducing) mastectomy. Ann Surg Oncol. 2017;24:375–97.
Domchek SM. Risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers: a complex discussion. JAMA. 2019;321:27.
Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–22.
Metcalfe K, Lynch HT, Foulkes WD, et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 2015;1:306–13.
Mersch J, Brown N, Pirzadeh-Miller S, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA. 2018;320:1266–74.
Wevers MR, Schmidt MK, Engelhardt EG, et al. Timing of risk reducing mastectomy in breast cancer patients carrying a BRCA1/2 mutation: retrospective data from the Dutch HEBON study. Fam Cancer. 2015;14:355–63.
Gill J, Obley AJ, Prasad V. Direct-to-consumer genetic testing: the implications of the US FDA’s first marketing authorization for BRCA mutation testing. JAMA. 2018;319:2377–8.
23andMe. https://www.23andme.com/?mdb1=true. Accessed 21 Apr 2019.
color. https://www.color.com/. Accessed 21 Apr 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors report no financial disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, J., Seng, S., Yoo, J. et al. Clinical Management of Patients at Risk for Hereditary Breast Cancer with Variants of Uncertain Significance in the Era of Multigene Panel Testing. Ann Surg Oncol 26, 3389–3396 (2019). https://doi.org/10.1245/s10434-019-07595-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07595-2