Abstract
Background
Hyperthermia enhances the cytotoxicity of chemotherapeutic agents used during cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC). However, this may result in an elevated core body temperature (CBT), with unintended effects on surgical morbidity. This study evaluates the relationship of maximum CBT during CRS/HIPEC on postoperative outcomes.
Methods
A retrospective review of patients undergoing CRS/HIPEC from January 2011 to July 2017 was performed. Outcomes were stratified according to maximum CBT reached during HIPEC. Primary study endpoints were 30-day morbidity and 30-day complication severity.
Results
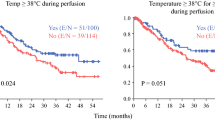
Overall, 135 consecutive CRS/HIPEC cases were reviewed; 36 (27%) had a maximum CBT ≥ 39.5 °C during the 90-min HIPEC. CBT ≥ 39.5 °C was associated with an increase in 30-day postoperative complications (58% vs. 34%, p = 0.01) and severe Clavien–Dindo grade III or higher complications (22% vs. 11%, p = 0.04). On multivariate analysis, the adjusted odds ratio of having any complication was 3.77 (95% confidence interval [CI] 1.56–9.14) and a Clavien–Dindo grade III or higher complication was 3.46 (95% CI 1.10–10.95) when maximum CBT reached 39.5 °C. Flow rates ≥ 2.35 L/min were associated with lower average CBT (p = 0.05) and improved peritoneal heating (p = 0.02).
Conclusion
Maximum CBT ≥ 39.5 °C is associated with an increased risk of postoperative morbidity. Higher flow rates are associated with improved intraperitoneal heating, lower CBT, and may contribute to optimizing the therapeutic benefit of HIPEC.
Similar content being viewed by others
References
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi- institutional study. J Clin Oncol. 2004;22:3284–92.
Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18.
Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev. 2016;48:42–9.
Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16:2152–65.
Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104-9.
Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31.
Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–62.
Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia. 2007;23:431–42.
Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–94.
Ohno S, Siddik ZH, Kido Y, Zwelling LA, Bull JMC. Thermal enhancement of drug uptake and DNA adducts as a possible mechanism for the effect of sequencing hyperthermia on cisplatin-induced cytotoxicity in L1210 cells. Cancer Chemother Pharmacol. 1994;34:302–6.
Song C, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005;21(8):761–768.
Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia. 2006;22(3):197–203.
Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528–42.
Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10:550–8.
Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56.
User Guide for the 2016 ACS NSQIP Participant Use Data File. American College of Surgeons National Surgical Quality Improvement Program; Oct 2017. pp. 15–8.
Dindo D, Demartines N, Clavien P. classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients. Ann Surg. 2004;240(2):205–13.
Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60.
Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion. Cancer Res. 1984;44:1840–2.
Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer. 1988;61:232–7.
Verwaal VJ, Bruin S, Boot H, et al. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32.
Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27(36):6237–42.
Chua T, Moran B, Sugarbaker P, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56.
Schaaf L, Kuip H, Zopf W, et al. A temperature of 40°C appears to be a critical threshold for potentiating cytotoxic chemotherapy in vitro and in peritoneal carcinomatosis patients undergoing HIPEC. Ann Surg Oncol. 2015;22:758–65.
Goldenshluger M, Zippel D, Ben-Yaacov A, et al. Core body temperature but not intraabdominal pressure predicts postoperative complications following closed system hyperthermic intraperitoneal chemotherapy (HIPEC) administration. Ann Surg Oncol. 2018;25:660–6.
Furman MJ, Picotte RJ, Wante MJ, et al. Higher flow rates improve heating during hyperthermic intraperitoneal chemoperfusion. J Surg Oncol. 2014;110:970–5.
Acknowledgment
The data for this research were provided by the Cancer Research Office of the University of Massachusetts Medical Center.
Funding
Funding for this study was provided by the Division of Surgical Oncology at the University of Massachusetts Medical School.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Ryan J. Hendrix, Jonathan P. Kassira, and Laura A. Lambert have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hendrix, R.J., Kassira, J.P. & Lambert, L.A. Elevated Maximum Core Body Temperature During Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) is Associated with Increased Postoperative Complications. Ann Surg Oncol 27, 232–239 (2020). https://doi.org/10.1245/s10434-019-07495-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07495-5