Abstract
Background
T cell factor 4 (TCF-4) mediates a nuclear response to wingless/int (Wnt) signals by interacting with β-catenin. Axis inhibition protein (axin) is an important negative regulator of the Wnt signaling pathway. Our aims were to examine the relationship between axin and TCF-4 and to explore the effects of axin on the development of lung cancer.
Methods
Expression levels of axin and TCF-4 were examined in 107 lung cancer specimens by immunohistochemistry. The axin gene was transfected into lung cancer BE1 cells. The expression levels of axin, β-catenin, and TCF-4 were detected with immunofluorescence and reverse transcription-polymerase chain reaction (RT-PCR) experiments. Apoptosis, proliferation, and the invasive ability of lung cancer cells were examined using flow cytometry, 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), and Matrigel invasive assays.
Results
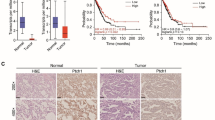
Preserved axin expression correlated negatively with TCF-4 expression (P = .031). Axin expression differed with respect to degree of differentiation (P = .025) and histological tumor type (P = .031). TCF-4 expression differed relative to tumor, node metastasis (TNM) stage (P = .024). BE1 cells transfected with axin (BE1-axin cells) exhibited a significant decrease in TCF-4 expression. The level of apoptosis in BE1-axin cells was significantly increased, while the proliferative and invasive abilities of BE1-axin cells were decreased.
Conclusion
These results suggest that reduced expression of axin or augmented expression of TCF-4 is associated with the malignant behavior of lung cancers. Overexpression of axin can downregulate expression of TCF-4 and can inhibit the ability of lung cancer cells to proliferate and invade.







Similar content being viewed by others
REFERENCES
Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 2003;129:199–221
Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol 2006;21:103–24
Ueta T, Ikeguchi M, Hirooka Y, Kaibara N, Terada T. Beta-catenin and cyclin D1 expression in human hepatocellular carcinoma. Oncol Rep 2002;9:1197–203
Gerstein AV, Almeida TA, Zhao G, et al. APC/CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer 2002;34:9–16
Cheng XX, Sun Y, Chen XY, Zhang KL, Kong QY, Liu J, Li H. Frequent translocalization of beta-catenin in gastric cancers and its relevance to tumor progression. Oncol Rep 2004;11:1201–7
Xu HT, Wang L, Lin D, Liu Y, Liu N, Yuan XM, Wang EH. Abnormal beta-catenin and reduced axin expression are associated with poor differentiation and progression in non-small cell lung cancer. Am J Clin Pathol 2006;125:534–41
Luo W, Lin SC. Axin: a master scaffold for multiple signaling pathways. Neurosignals 2004;13:99–113
Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol 2005;58:225–36
Neo SY, Zhang Y, Yaw LP, Li P, Lin SC. Axin-induced apoptosis depends on the extent of its JNK activation and its ability to down-regulate beta-catenin levels. Biochem Biophys Res Commun 2000;272:144–50
Furuhashi M, Yagi K, Yamamoto H, et al. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol 2001;21:5132–41
Nakajima M, Fukuchi M, Miyazaki T, Masuda N, Kato H, Kuwano H. Reduced expression of Axin correlates with tumour progression of oesophageal squamous cell carcinoma. Br J Cancer 2003;88:1734–9
Rui Y, Xu Z, Lin S, et al. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J 2004;23:4583–94
Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumors of the lung, pleura, thymus and heart. World Health Organization Classification of Tumors. Lyon: IARC Press, 2004
Watanabe Y. TNM classification for lung cancer. Ann Thorac Cardiovasc Surg 2003;9:343–50
Li CY, Wang Y, Cui ZS, Wang EH. Expression of T cell factor-4 in non-small-cell lung cancer. Chin Med J (Engl) 2005;118:136–40
Liu CR, Ma CS, Ning JY, You JF, Liao SL, Zheng J. Differential thymosin beta 10 expression levels and actin filament organization in tumor cell lines with different metastatic potential. Chin Med J (Engl) 2004;117:213–18
Fukumoto S, Hsieh CM, Maemura K, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 2001;276:17479–83
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991;139:271–9
Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res 1998;58:1130–4
Park JY, Park WS, Nam SW, et al. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int 2005;25:70–6
Clements WM, Wang J, Sarnaik A, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 2002;62:3503–6
Ueda M, Gemmill RM, West J, et al. Mutations of the beta- and gamma-catenin genes are uncommon in human lung, breast, kidney, cervical and ovarian carcinomas. Br J Cancer 2001;85:64–8
Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y. Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J Cancer Res Clin Oncol 2005;131:773–82
Su MC, Wang CC, Chen CC, et al. Nuclear translocation of beta-catenin protein but absence of beta-catenin and APC mutation in gastrointestinal carcinoid tumor. Ann Surg Oncol 2006;13:1604–9
Zeng G, Germinaro M, Micsenyi A, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia 2006;8:279–89
Zhou CX, Gao Y. Frequent genetic alterations and reduced expression of the Axin1 gene in oral squamous cell carcinoma: involvement in tumor progression and metastasis. Oncol Rep 2007;17:73–9
Rother K, Johne C, Spiesbach K, et al. Identification of Tcf-4 as a transcriptional target of p53 signalling. Oncogene 2004;23:3376–84
Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Upregulation of TCF4 expression as a transcriptional target of beta-catenin/p300 complexes during trans-differentiation of endometrial carcinoma cells. Lab Invest 2005;85:768–79
Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999;154:515–23
Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol 2004;35:211–31
ACKNOWLEDGMENTS
We thank Dr. Jie Zheng at Medical College of Beijing University, China for providing lung cancer BE1 cell lines. We also thank Professor Mark A. Perrella at Brigham & Women’s Hospital, Boston, MA, USA for kindly providing axin expression vector pFLAG-CMV-5b-axin containing mouse axin1 gene.
This study was supported by National Natural Science Foundation of China (Grants 30470764 and 30670917 to E.-H. Wang) and Doctoral Fund of Ministry of Education of China (No.[2004]165 to E.-H. Wang).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, HT., Wei, Q., Liu, Y. et al. Overexpression of Axin Downregulates TCF-4 and Inhibits the Development of Lung Cancer. Ann Surg Oncol 14, 3251–3259 (2007). https://doi.org/10.1245/s10434-007-9555-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9555-9