-
PDF
- Split View
-
Views
-
Cite
Cite
Yuichi Yoshida, Rika Yoshida, Kanako Shibuta, Yoshinori Ozeki, Mitsuhiro Okamoto, Koro Gotoh, Takayuki Masaki, Hirotaka Shibata, Quality of Life of Primary Aldosteronism Patients by Mineralocorticoid Receptor Antagonists, Journal of the Endocrine Society, Volume 5, Issue 4, April 2021, bvab020, https://doi.org/10.1210/jendso/bvab020
Close - Share Icon Share
Abstract
Although primary aldosteronism (PA) reduces quality of life (QOL), there have been no reports on whether treatment with a mineralocorticoid receptor antagonist (MRA) improves QOL in Japanese PA patients.
Using the 36-Item Short-Form Health Survey (SF-36), we compared the QOL of PA patients before and after treatment and evaluated whether the effectiveness of MRAs differs by sex and serum potassium level.
In 50 patients diagnosed with PA (with or without hypokalemia) and treated with an MRA, the SF-36 scores, blood pressure, and clinical features were assessed before, and 3 and 6 months after treatment. Separate analyses were also conducted for males and females.
The normative mean SF-36 score of the healthy subjects was 50. The pretreatment Role-Physical (RP) (46.7 ± 1.8, P = .019), General Health (47.1 ± 1.3, P = .042), and Role-Emotional (47.2 ± 1.7, P = .045) SF-36 subscale scores of all PA patients were significantly lower than those of healthy subjects but were improved by MRA treatment. Females with PA had a lower RP score (45.1 ± 2.2, P = .008), which was not improved by MRA treatment (46.1 ± 2.4, P = .036). In addition, PA patients with hypokalemia had a lower Mental Health SF-36 subscale score (43.2 ± 4.4, P = .041), which was improved by treatment with an MRA.
MRAs improved the QOL of Japanese PA patients, but female PA patients may be more resistant to MRAs.
Primary aldosteronism (PA) is a common disease, with an incidence of approximately 5% to 15% in hypertensive patients [1-3]. PA may cause hypertension and hypokalemia due to aldosterone overproduction by the adrenal gland, as well as cerebral infarction and myocardial infarction [4]. The global standard treatment is adrenalectomy for unilateral PA, and mineralocorticoid receptor antagonist (MRA) treatment for bilateral PA [5, 6]. Sonino et al. [7] reported that patients with PA had a higher prevalence of anxiety disorders than healthy individuals, and other reports have shown that quality of life (QOL) is affected in PA patients [8-14].
The Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) is a globally recognized QOL questionnaire, with 8 mental and physical QOL subscales. SF-36 data are easy to compare between clinical and healthy populations because normative data from healthy individuals have already been obtained, including data from 2279 healthy Japanese people [15-17]. Some studies of the psychological impact of PA on QOL using the SF-36 or similar questionnaires have reported that PA patients have a lower QOL, which improves with adrenalectomy or MRA treatment [8, 9, 11-14]. However, most of these reports were on adrenalectomy for unilateral PA, and only 3 reports have assessed QOL after treatment with an MRA [9, 11, 12]. Of these 3 reports, none were on MRA treatment of Asian PA patients; therefore, it is unknown whether this treatment improves QOL in Japanese PA patients. In addition, although there have been reports of QOL in PA patients stratified by sex [11, 18], QOL was not reported in terms of clinical severity.
In this study, we used the SF-36 to determine whether QOL improved in Japanese PA patients after treatment with an MRA. Furthermore, we examined QOL changes by sex and hypokalemia status.
Patients and Methods
Subjects
We prospectively enrolled 72 patients diagnosed with PA at Oita University Hospital (Oita, Japan) between July 1, 2017, and May 31, 2020, and who agreed to complete the SF-36. Of the 72 patients, 56 completed the SF-36 before treatment, and at 3 and 6 months after treatment; 16 patients who did not complete the questionnaire were excluded. A further 6 patients were excluded because they were diagnosed with unilateral PA by adrenal vein sampling (AVS) and underwent adrenalectomy; the remaining 50 patients, all of whom were given an MRA, were examined. AVS was performed in 45 of the 50 patients; the remaining 5 did not want to undergo adrenalectomy or had an allergy to a contrast agent. Thirty-seven patients were diagnosed with bilateral PA, and catheter insertion failed in 6. Two patients were diagnosed with unilateral PA but were treated with an MRA at their own request. This study was approved by the Oita University Ethics Committee and complied with the Declaration of Helsinki.
PA Diagnosis
Patients were diagnosed with PA according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension [6]. Screening tests were performed by measuring the active renin concentration (ARC) and plasma aldosterone concentration (PAC) in the morning. The screening test was considered positive when the PAC (pg/mL)/ARC (pg/mL) ratio (ARR) was >40. Subsequently, the saline infusion test, captopril challenge test, and oral salt loading test were performed as confirmatory tests. The saline infusion test was performed by intravenous infusion of 2 L of saline over 4 hours in the supine position. When the PAC was >60 pg/mL after saline loading, the test was considered positive. The captopril challenge test was performed by administration of 50-mg captopril tablets, and blood was drawn after 90 minutes. When the PAC/ARC ratio was >40 at 90 minutes after administration, the test was considered positive. The oral salt loading test was performed on 24-hour urine samples after consumption of a high salt diet. The test was considered positive when the 24-hour urinary aldosterone and sodium (Na) excretion amounts were >8 μg/day and >170 mEq/day, respectively. When urine Na was <170 mEq/day, the data were excluded due to insufficient sodium chloride loading. Because angiotensin receptor blockers, MRAs, beta-adrenergic blockers, and diuretics affect renin and aldosterone, they were discontinued or changed to calcium channel blockers or alpha-adrenergic blockers before the confirmatory test. When AVS was performed, catheter insertion was considered to be successful when the adrenal vein–inferior vena cava cortisol step-up was higher than 5-fold in the presence of adrenocorticotropic hormone stimulation. Unilateral PA was diagnosed when the “high-side adrenal vein aldosterone to cortisol ratio,” which was divided by the “low-side adrenal vein aldosterone to cortisol ratio,” was higher than 4 (lateralized ratio). In addition, when the low-side adrenal vein aldosterone to cortisol ratio, which was divided by the inferior vena cava aldosterone to cortisol ratio, was <1 (contralateral ratio), unilateral lesion (high side) was indicated.
Medical Treatment
Patients diagnosed with bilateral PA using the above method, those who failed catheterization with AVS, or who did not wish to have adrenalectomy were orally administered either spironolactone, eplerenone, or esaxerenone as an MRA. After starting the MRA, the dose was adjusted appropriately with reference to blood pressure, potassium (K), and renin levels, but was not discontinued. Before the start of MRA treatment, the blood pressure was measured after hospitalization and bed rest. At both 3 and 6 months after the start of treatment, blood pressure was measured 3 times at home, with the mean values used in the analysis. Concomitant with MRAs, calcium channel blockers, and alpha-adrenergic blockers were continued.
Evaluation of QOL by the SF-36
The SF-36 is a self-report instrument. Completed SF-36 questionnaires were collected from the participants before the start of treatment (after the diagnosis of PA), and at 3 and 6 months after beginning MRA treatment. The normative mean ± SD SF-36 score, namely that for healthy subjects, was taken as 50 ± 10. The 8 SF-36 subscales are Physical Functioning (PF), Role-Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE), and Mental Health (MH).
Laboratory Tests
Blood was sampled in the morning. PAC was measured by chemiluminescent enzyme immunoassay (CLEIA; Accuraseed® Aldosterone Kit; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), ARC was measured only at baseline by CLEIA (Accuraseed® Renin Kit; FUJIFILM Wako Pure Chemical Corporation), and urine aldosterone was measured using the radioimmunoassay Spac S® Aldosterone Kit (TFB Corporation, Tokyo, Japan).
Statistical Analysis
Values of ARC, PAC, and ARR are expressed as the median (25th percentile to 75th percentile), and the other values are expressed as the mean ± standard error of the mean (SEM). For comparison between healthy subjects (n = 2279) and PA patients, the z-test was performed using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA). The 2-sided unpaired t-test and analysis of variance were used to compare 2 and 3 groups, respectively, with the analyses performed with GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). P < .05 was considered statistically significant.
Results
Clinical Features and SF-36 Scores Before Treatment, and at 3 and 6 Months After Treatment With an MRA
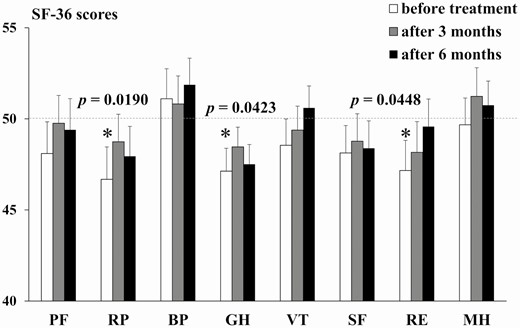
There were 21 males and 29 females in this study. The mean age, body mass index, and number of antihypertensive drugs excluding MRA was 55.7 ± 12.6 years, 22.2 ± 4.4 kg/m2, and 0.86, respectively. The SF-36 scores at the various time points are shown in Fig. 1 and Table 1, and clinical features according to MRA treatment status are shown in Table 2. Eplerenone was the most used MRA. One male who started treatment with spironolactone was switched to esaxerenone at 3 months after treatment due to the appearance of gynecomastia symptoms. Regarding the SF-36 subscale scores, PA patients before treatment showed lower RP, GH, and RE scores (ie, lower QOL in these domains) than healthy subjects. The RP, GH, and RE scores were improved to levels that were similar to healthy subjects at 3 and 6 months after treatment. No difference in scores in the other 5 SF-36 domains was observed between PA patients and healthy subjects at any time point. Regarding other clinical features, systolic blood pressure (SBP) was significantly decreased, whereas serum K and ARC were significantly increased, in PA patients at 6 months after treatment compared with pretreatment levels. An average of 0.86 antihypertensive agents, excluding MRAs, were taken before treatment with MRAs, which had not changed for 6 months.
Clinical features of PA patients before and 3 and 6 months after treatment with an MRA
| . | baseline . | 3 months . | baseline vs 3 months (P value) . | 6 months . | baseline vs 3 months (P value) . |
|---|---|---|---|---|---|
| SBP (mmHg) | 140.6 ± 2.5 | 128.0 ± 1.9 | <.001 | 125.1 ± 1.2 | <.001 |
| DBP (mmHg) | 85.0 ± 1.9 | 81.9 ± 1.5 | .401 | 79.8 ± 1.5 | .079 |
| K (mEq/L) | 3.8 ± 0.1 | 4.3 ± 0.1 | <.001 | 4.2 ± 0.1 | <.001 |
| ARC (pg/mL) | 2.7 (1.1–5.5) | 4.5 (1.9–10.4) | .054 | 5.1 (3.5–10.2) | .002 |
| PAC (pg/mL) | 191.2 (147.0–235.5) | 212.6 (151.8–296.0) | .783 | 213.5 (130.5–312.1) | .580 |
| ARR (PAC/ARC) | 63.6 (44.8–155.5) | 38.7 (19.8–85.9) | .321 | 33.0 (20.1–59.9) | .050 |
| MRA | |||||
| Spironolactone (N) | 14 | 13 | |||
| Dose (mg) | 28.6 ± 2.4 | 28.8 ± 2.6 | |||
| Eplerenone (N) | 30 | 30 | |||
| Dose (mg) | 42.9 ± 2.6 | 42.9 ± 2.6 | |||
| Esaxerenone (N) | 6 | 7 | |||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 |
| . | baseline . | 3 months . | baseline vs 3 months (P value) . | 6 months . | baseline vs 3 months (P value) . |
|---|---|---|---|---|---|
| SBP (mmHg) | 140.6 ± 2.5 | 128.0 ± 1.9 | <.001 | 125.1 ± 1.2 | <.001 |
| DBP (mmHg) | 85.0 ± 1.9 | 81.9 ± 1.5 | .401 | 79.8 ± 1.5 | .079 |
| K (mEq/L) | 3.8 ± 0.1 | 4.3 ± 0.1 | <.001 | 4.2 ± 0.1 | <.001 |
| ARC (pg/mL) | 2.7 (1.1–5.5) | 4.5 (1.9–10.4) | .054 | 5.1 (3.5–10.2) | .002 |
| PAC (pg/mL) | 191.2 (147.0–235.5) | 212.6 (151.8–296.0) | .783 | 213.5 (130.5–312.1) | .580 |
| ARR (PAC/ARC) | 63.6 (44.8–155.5) | 38.7 (19.8–85.9) | .321 | 33.0 (20.1–59.9) | .050 |
| MRA | |||||
| Spironolactone (N) | 14 | 13 | |||
| Dose (mg) | 28.6 ± 2.4 | 28.8 ± 2.6 | |||
| Eplerenone (N) | 30 | 30 | |||
| Dose (mg) | 42.9 ± 2.6 | 42.9 ± 2.6 | |||
| Esaxerenone (N) | 6 | 7 | |||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 |
Data of PAC, ARC, and ARR are expressed as the median (25th–75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
Clinical features of PA patients before and 3 and 6 months after treatment with an MRA
| . | baseline . | 3 months . | baseline vs 3 months (P value) . | 6 months . | baseline vs 3 months (P value) . |
|---|---|---|---|---|---|
| SBP (mmHg) | 140.6 ± 2.5 | 128.0 ± 1.9 | <.001 | 125.1 ± 1.2 | <.001 |
| DBP (mmHg) | 85.0 ± 1.9 | 81.9 ± 1.5 | .401 | 79.8 ± 1.5 | .079 |
| K (mEq/L) | 3.8 ± 0.1 | 4.3 ± 0.1 | <.001 | 4.2 ± 0.1 | <.001 |
| ARC (pg/mL) | 2.7 (1.1–5.5) | 4.5 (1.9–10.4) | .054 | 5.1 (3.5–10.2) | .002 |
| PAC (pg/mL) | 191.2 (147.0–235.5) | 212.6 (151.8–296.0) | .783 | 213.5 (130.5–312.1) | .580 |
| ARR (PAC/ARC) | 63.6 (44.8–155.5) | 38.7 (19.8–85.9) | .321 | 33.0 (20.1–59.9) | .050 |
| MRA | |||||
| Spironolactone (N) | 14 | 13 | |||
| Dose (mg) | 28.6 ± 2.4 | 28.8 ± 2.6 | |||
| Eplerenone (N) | 30 | 30 | |||
| Dose (mg) | 42.9 ± 2.6 | 42.9 ± 2.6 | |||
| Esaxerenone (N) | 6 | 7 | |||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 |
| . | baseline . | 3 months . | baseline vs 3 months (P value) . | 6 months . | baseline vs 3 months (P value) . |
|---|---|---|---|---|---|
| SBP (mmHg) | 140.6 ± 2.5 | 128.0 ± 1.9 | <.001 | 125.1 ± 1.2 | <.001 |
| DBP (mmHg) | 85.0 ± 1.9 | 81.9 ± 1.5 | .401 | 79.8 ± 1.5 | .079 |
| K (mEq/L) | 3.8 ± 0.1 | 4.3 ± 0.1 | <.001 | 4.2 ± 0.1 | <.001 |
| ARC (pg/mL) | 2.7 (1.1–5.5) | 4.5 (1.9–10.4) | .054 | 5.1 (3.5–10.2) | .002 |
| PAC (pg/mL) | 191.2 (147.0–235.5) | 212.6 (151.8–296.0) | .783 | 213.5 (130.5–312.1) | .580 |
| ARR (PAC/ARC) | 63.6 (44.8–155.5) | 38.7 (19.8–85.9) | .321 | 33.0 (20.1–59.9) | .050 |
| MRA | |||||
| Spironolactone (N) | 14 | 13 | |||
| Dose (mg) | 28.6 ± 2.4 | 28.8 ± 2.6 | |||
| Eplerenone (N) | 30 | 30 | |||
| Dose (mg) | 42.9 ± 2.6 | 42.9 ± 2.6 | |||
| Esaxerenone (N) | 6 | 7 | |||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 |
Data of PAC, ARC, and ARR are expressed as the median (25th–75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in PA patients compared with the general Japanese population
| . | SF-36 scores . | P values . | ||||
|---|---|---|---|---|---|---|
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 48.1 ± 1.7 | 49.8 ± 1.5 | 49.4 ± 1.7 | .179 | .864 | .670 |
| RP | 46.7 ± 1.8 | 48.7 ± 1.5 | 47.9 ± 1.6 | .019 | .374 | .146 |
| BP | 51.1 ± 1.6 | 50.8 ± 1.5 | 51.9 ± 1.5 | .436 | .564 | .186 |
| GH | 47.1 ± 1.3 | 48.5 ± 1.1 | 47.5 ± 1.1 | .042 | .276 | .079 |
| VT | 48.5 ± 1.4 | 49.4 ± 1.3 | 50.6 ± 1.2 | .304 | .662 | .670 |
| SF | 48.1 ± 1.5 | 48.8 ± 1.5 | 48.4 ± 1.5 | .186 | .385 | .254 |
| RE | 47.2 ± 1.7 | 48.2 ± 1.7 | 49.6 ± 1.5 | .045 | .194 | .765 |
| MH | 49.7 ± 1.5 | 51.2 ± 1.5 | 50.7 ± 1.3 | .818 | .384 | .597 |
| . | SF-36 scores . | P values . | ||||
|---|---|---|---|---|---|---|
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 48.1 ± 1.7 | 49.8 ± 1.5 | 49.4 ± 1.7 | .179 | .864 | .670 |
| RP | 46.7 ± 1.8 | 48.7 ± 1.5 | 47.9 ± 1.6 | .019 | .374 | .146 |
| BP | 51.1 ± 1.6 | 50.8 ± 1.5 | 51.9 ± 1.5 | .436 | .564 | .186 |
| GH | 47.1 ± 1.3 | 48.5 ± 1.1 | 47.5 ± 1.1 | .042 | .276 | .079 |
| VT | 48.5 ± 1.4 | 49.4 ± 1.3 | 50.6 ± 1.2 | .304 | .662 | .670 |
| SF | 48.1 ± 1.5 | 48.8 ± 1.5 | 48.4 ± 1.5 | .186 | .385 | .254 |
| RE | 47.2 ± 1.7 | 48.2 ± 1.7 | 49.6 ± 1.5 | .045 | .194 | .765 |
| MH | 49.7 ± 1.5 | 51.2 ± 1.5 | 50.7 ± 1.3 | .818 | .384 | .597 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in PA patients compared with the general Japanese population
| . | SF-36 scores . | P values . | ||||
|---|---|---|---|---|---|---|
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 48.1 ± 1.7 | 49.8 ± 1.5 | 49.4 ± 1.7 | .179 | .864 | .670 |
| RP | 46.7 ± 1.8 | 48.7 ± 1.5 | 47.9 ± 1.6 | .019 | .374 | .146 |
| BP | 51.1 ± 1.6 | 50.8 ± 1.5 | 51.9 ± 1.5 | .436 | .564 | .186 |
| GH | 47.1 ± 1.3 | 48.5 ± 1.1 | 47.5 ± 1.1 | .042 | .276 | .079 |
| VT | 48.5 ± 1.4 | 49.4 ± 1.3 | 50.6 ± 1.2 | .304 | .662 | .670 |
| SF | 48.1 ± 1.5 | 48.8 ± 1.5 | 48.4 ± 1.5 | .186 | .385 | .254 |
| RE | 47.2 ± 1.7 | 48.2 ± 1.7 | 49.6 ± 1.5 | .045 | .194 | .765 |
| MH | 49.7 ± 1.5 | 51.2 ± 1.5 | 50.7 ± 1.3 | .818 | .384 | .597 |
| . | SF-36 scores . | P values . | ||||
|---|---|---|---|---|---|---|
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 48.1 ± 1.7 | 49.8 ± 1.5 | 49.4 ± 1.7 | .179 | .864 | .670 |
| RP | 46.7 ± 1.8 | 48.7 ± 1.5 | 47.9 ± 1.6 | .019 | .374 | .146 |
| BP | 51.1 ± 1.6 | 50.8 ± 1.5 | 51.9 ± 1.5 | .436 | .564 | .186 |
| GH | 47.1 ± 1.3 | 48.5 ± 1.1 | 47.5 ± 1.1 | .042 | .276 | .079 |
| VT | 48.5 ± 1.4 | 49.4 ± 1.3 | 50.6 ± 1.2 | .304 | .662 | .670 |
| SF | 48.1 ± 1.5 | 48.8 ± 1.5 | 48.4 ± 1.5 | .186 | .385 | .254 |
| RE | 47.2 ± 1.7 | 48.2 ± 1.7 | 49.6 ± 1.5 | .045 | .194 | .765 |
| MH | 49.7 ± 1.5 | 51.2 ± 1.5 | 50.7 ± 1.3 | .818 | .384 | .597 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
Pre- and post-treatment SF-36 scores of all PA patients on an MRA. SF-36 subscale scores before treatment and at 3 and 6 months after treatment with an MRA. The normative mean ± standard deviation SF-36 score, ie, that for healthy subjects, was taken as 50 ±10. *P < .05 compared with the general Japanese population. Bar shows the SEM. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
SF-36 Scores and Clinical Features of PA Patients by Sex
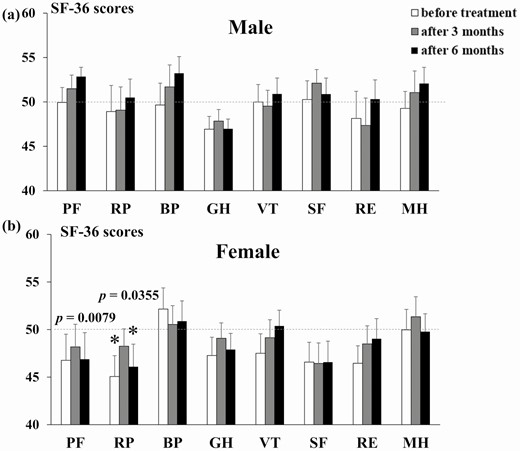
The mean age, body mass index, and number of antihypertensive drugs excluding MRA of males and females were 53.6 ± 2.9 and 57.2 ± 2.2 years, 22.9 ± 1.2 and 21.6 ± 0.6 kg/m2, and 0.86 and 0.86, respectively. The SF-36 scores over time by sex are shown in Fig. 2 and Table 3; the clinical features are shown in Table 4. The SF-36 score did not differ between male PA patients and healthy subjects, whereas SBP and diastolic blood pressure (DBP) were decreased, and the K level and ARC were increased, at 6 months after the start of MRA treatment compared with pretreatment levels. In female PA patients, both the pretreatment and 6-month after MRA treatment RP scores were lower than healthy subjects. Moreover, the SBP decreased, and serum K increased, at 6 months after treatment in the female PA patients, while no significant changes in DBP or ARC were seen at 6 months compared with pretreatment. Both SBP and DBP were higher in male than female patients with PA before treatment, but there was no significant difference at 6 months after treatment. There was no difference in ARC between the male and female patients with PA before treatment, but the ARC was significantly lower in females at 6 months after treatment. There was no difference in MRA dose between males and females.
Clinical characteristics of male and female PA patients, before and 3 and 6 months after, treatment with an MRA
| . | Male . | Female . | Male vs female P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 21 . | . | . | . | . | 29 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 149.0 ± 3.5 | 132.3 ± 2.8 | <.001 | 127.1 ± 1.7 | <.001 | 134.7 ± 2.9 | 123.8 ± 2.2 | .008 | 122.8 ± 1.6 | .002 | .003 | .023 | .079 |
| DBP (mmHg) | 90.5 ± 2.9 | 85.1 ± 2.0 | .234 | 80.7 ± 2.0 | .014 | 81.0 ± 2.3 | 78.5 ± 2.0 | .689 | 79.6 ± 1.8 | .880 | .014 | .025 | .683 |
| K (mEq/L) | 3.7 ± 0.1 | 4.4 ± 0.1 | <.001 | 4.3 ± 0.1 | .001 | 3.9 ± 0.1 | 4.2 ± 0.1 | .019 | 4.2 ± 0.1 | .005 | .278 | .042 | .554 |
| ARC (pg/mL) | 2.9 (1.7–5.8) | 6.2 (4.0–13.5) | .170 | 7.4 (4.8–13.3) | .010 | 1.9 (0.9–5.2) | 3.4 (1.6–9.0) | .141 | 4.1 (2.5–5.2) | .141 | .335 | .083 | .021 |
| PAC (pg/mL) | 200.5 (165.8–235.5) | 207.7 (130.9–268.5) | .990 | 202.9 (154.7–286.1) | .669 | 186.0 (142.0–238.5) | 218.9 (172.0–303.8) | .462 | 235.9 (131.6–325.1) | .414 | .495 | .547 | .651 |
| ARR (PAC/ARC) | 58.8 (49.2–104.7) | 30.4 (18.2–45.4) | .061 | 28.1 (15.7–35.0) | .057 | 99.0 (38.3–250.8) | 56.2 (31.6–122.4) | .840 | 47.0 (26.1–80.0) | .258 | .209 | .051 | .078 |
| MRA | |||||||||||||
| Spironolactone (N) | 8 | 7 | 6 | 6 | |||||||||
| Dose (mg) | 28.1 ± 3.1 | 28.6 ± 3.6 | 29.2 ± 4.2 | 29.2 ± 4.2 | |||||||||
| Eplerenone (N) | 9 | 9 | 21 | 21 | |||||||||
| Dose (mg) | 43.8 ± 6.3 | 43.8 ± 6.3 | 42.5 ± 4.1 | 45.0 ± 5.0 | |||||||||
| Esaxerenone (N) | 4 | 5 | 2 | 2 | |||||||||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
| . | Male . | Female . | Male vs female P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 21 . | . | . | . | . | 29 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 149.0 ± 3.5 | 132.3 ± 2.8 | <.001 | 127.1 ± 1.7 | <.001 | 134.7 ± 2.9 | 123.8 ± 2.2 | .008 | 122.8 ± 1.6 | .002 | .003 | .023 | .079 |
| DBP (mmHg) | 90.5 ± 2.9 | 85.1 ± 2.0 | .234 | 80.7 ± 2.0 | .014 | 81.0 ± 2.3 | 78.5 ± 2.0 | .689 | 79.6 ± 1.8 | .880 | .014 | .025 | .683 |
| K (mEq/L) | 3.7 ± 0.1 | 4.4 ± 0.1 | <.001 | 4.3 ± 0.1 | .001 | 3.9 ± 0.1 | 4.2 ± 0.1 | .019 | 4.2 ± 0.1 | .005 | .278 | .042 | .554 |
| ARC (pg/mL) | 2.9 (1.7–5.8) | 6.2 (4.0–13.5) | .170 | 7.4 (4.8–13.3) | .010 | 1.9 (0.9–5.2) | 3.4 (1.6–9.0) | .141 | 4.1 (2.5–5.2) | .141 | .335 | .083 | .021 |
| PAC (pg/mL) | 200.5 (165.8–235.5) | 207.7 (130.9–268.5) | .990 | 202.9 (154.7–286.1) | .669 | 186.0 (142.0–238.5) | 218.9 (172.0–303.8) | .462 | 235.9 (131.6–325.1) | .414 | .495 | .547 | .651 |
| ARR (PAC/ARC) | 58.8 (49.2–104.7) | 30.4 (18.2–45.4) | .061 | 28.1 (15.7–35.0) | .057 | 99.0 (38.3–250.8) | 56.2 (31.6–122.4) | .840 | 47.0 (26.1–80.0) | .258 | .209 | .051 | .078 |
| MRA | |||||||||||||
| Spironolactone (N) | 8 | 7 | 6 | 6 | |||||||||
| Dose (mg) | 28.1 ± 3.1 | 28.6 ± 3.6 | 29.2 ± 4.2 | 29.2 ± 4.2 | |||||||||
| Eplerenone (N) | 9 | 9 | 21 | 21 | |||||||||
| Dose (mg) | 43.8 ± 6.3 | 43.8 ± 6.3 | 42.5 ± 4.1 | 45.0 ± 5.0 | |||||||||
| Esaxerenone (N) | 4 | 5 | 2 | 2 | |||||||||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
Data of PAC, ARC, and ARR are expressed as the median (25th percentile-75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
aP values for comparison of male data at baseline.
bP values for comparison of female data at baseline.
Clinical characteristics of male and female PA patients, before and 3 and 6 months after, treatment with an MRA
| . | Male . | Female . | Male vs female P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 21 . | . | . | . | . | 29 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 149.0 ± 3.5 | 132.3 ± 2.8 | <.001 | 127.1 ± 1.7 | <.001 | 134.7 ± 2.9 | 123.8 ± 2.2 | .008 | 122.8 ± 1.6 | .002 | .003 | .023 | .079 |
| DBP (mmHg) | 90.5 ± 2.9 | 85.1 ± 2.0 | .234 | 80.7 ± 2.0 | .014 | 81.0 ± 2.3 | 78.5 ± 2.0 | .689 | 79.6 ± 1.8 | .880 | .014 | .025 | .683 |
| K (mEq/L) | 3.7 ± 0.1 | 4.4 ± 0.1 | <.001 | 4.3 ± 0.1 | .001 | 3.9 ± 0.1 | 4.2 ± 0.1 | .019 | 4.2 ± 0.1 | .005 | .278 | .042 | .554 |
| ARC (pg/mL) | 2.9 (1.7–5.8) | 6.2 (4.0–13.5) | .170 | 7.4 (4.8–13.3) | .010 | 1.9 (0.9–5.2) | 3.4 (1.6–9.0) | .141 | 4.1 (2.5–5.2) | .141 | .335 | .083 | .021 |
| PAC (pg/mL) | 200.5 (165.8–235.5) | 207.7 (130.9–268.5) | .990 | 202.9 (154.7–286.1) | .669 | 186.0 (142.0–238.5) | 218.9 (172.0–303.8) | .462 | 235.9 (131.6–325.1) | .414 | .495 | .547 | .651 |
| ARR (PAC/ARC) | 58.8 (49.2–104.7) | 30.4 (18.2–45.4) | .061 | 28.1 (15.7–35.0) | .057 | 99.0 (38.3–250.8) | 56.2 (31.6–122.4) | .840 | 47.0 (26.1–80.0) | .258 | .209 | .051 | .078 |
| MRA | |||||||||||||
| Spironolactone (N) | 8 | 7 | 6 | 6 | |||||||||
| Dose (mg) | 28.1 ± 3.1 | 28.6 ± 3.6 | 29.2 ± 4.2 | 29.2 ± 4.2 | |||||||||
| Eplerenone (N) | 9 | 9 | 21 | 21 | |||||||||
| Dose (mg) | 43.8 ± 6.3 | 43.8 ± 6.3 | 42.5 ± 4.1 | 45.0 ± 5.0 | |||||||||
| Esaxerenone (N) | 4 | 5 | 2 | 2 | |||||||||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
| . | Male . | Female . | Male vs female P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 21 . | . | . | . | . | 29 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 149.0 ± 3.5 | 132.3 ± 2.8 | <.001 | 127.1 ± 1.7 | <.001 | 134.7 ± 2.9 | 123.8 ± 2.2 | .008 | 122.8 ± 1.6 | .002 | .003 | .023 | .079 |
| DBP (mmHg) | 90.5 ± 2.9 | 85.1 ± 2.0 | .234 | 80.7 ± 2.0 | .014 | 81.0 ± 2.3 | 78.5 ± 2.0 | .689 | 79.6 ± 1.8 | .880 | .014 | .025 | .683 |
| K (mEq/L) | 3.7 ± 0.1 | 4.4 ± 0.1 | <.001 | 4.3 ± 0.1 | .001 | 3.9 ± 0.1 | 4.2 ± 0.1 | .019 | 4.2 ± 0.1 | .005 | .278 | .042 | .554 |
| ARC (pg/mL) | 2.9 (1.7–5.8) | 6.2 (4.0–13.5) | .170 | 7.4 (4.8–13.3) | .010 | 1.9 (0.9–5.2) | 3.4 (1.6–9.0) | .141 | 4.1 (2.5–5.2) | .141 | .335 | .083 | .021 |
| PAC (pg/mL) | 200.5 (165.8–235.5) | 207.7 (130.9–268.5) | .990 | 202.9 (154.7–286.1) | .669 | 186.0 (142.0–238.5) | 218.9 (172.0–303.8) | .462 | 235.9 (131.6–325.1) | .414 | .495 | .547 | .651 |
| ARR (PAC/ARC) | 58.8 (49.2–104.7) | 30.4 (18.2–45.4) | .061 | 28.1 (15.7–35.0) | .057 | 99.0 (38.3–250.8) | 56.2 (31.6–122.4) | .840 | 47.0 (26.1–80.0) | .258 | .209 | .051 | .078 |
| MRA | |||||||||||||
| Spironolactone (N) | 8 | 7 | 6 | 6 | |||||||||
| Dose (mg) | 28.1 ± 3.1 | 28.6 ± 3.6 | 29.2 ± 4.2 | 29.2 ± 4.2 | |||||||||
| Eplerenone (N) | 9 | 9 | 21 | 21 | |||||||||
| Dose (mg) | 43.8 ± 6.3 | 43.8 ± 6.3 | 42.5 ± 4.1 | 45.0 ± 5.0 | |||||||||
| Esaxerenone (N) | 4 | 5 | 2 | 2 | |||||||||
| Dose (mg) | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
Data of PAC, ARC, and ARR are expressed as the median (25th percentile-75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
aP values for comparison of male data at baseline.
bP values for comparison of female data at baseline.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in male and female PA patients compared with the general Japanese population
| . | Male . | Female . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 49.9 ± 1.7 | 51.5 ± 1.5 | 52.9 ± 1.0 | .977 | .496 | .190 | 46.8 ± 2.7 | 48.5 ± 2.4 | 46.9 ± 2.8 | .082 | .422 | .094 |
| RP | 48.9 ± 2.9 | 49.1 ± 2.6 | 50.5 ± 2.1 | .620 | .672 | .819 | 45.1 ± 2.2 | 48.5 ± 1.8 | 46.1 ± 2.4 | .008 | .420 | .036 |
| BP | 49.7 ± 2.5 | 51.7 ± 2.5 | 53.2 ± 1.9 | .875 | .437 | .139 | 52.1 ± 2.2 | 50.2 ± 2.0 | 50.9 ± 2.1 | .247 | .923 | .633 |
| GH | 46.9 ± 1.4 | 47.8 ± 1.3 | 47.0 ± 1.1 | .159 | .322 | .166 | 47.3 ± 1.9 | 48.9 ± 1.6 | 47.9 ± 1.7 | .142 | .557 | .258 |
| VT | 50.0 ± 2.0 | 49.5 ± 1.8 | 50.9 ± 1.8 | .994 | .828 | .679 | 47.5 ± 2.0 | 49.3 ± 1.9 | 50.4 ± 1.6 | .179 | .697 | .836 |
| SF | 50.3 ± 2.1 | 52.1 ± 1.5 | 50.9 ± 1.8 | .902 | .334 | .686 | 46.6 ± 2.1 | 46.8 ± 2.2 | 46.6 ± 2.2 | .065 | .085 | .065 |
| RE | 48.1 ± 3.1 | 47.3 ± 3.1 | 50.3 ± 2.2 | .394 | .224 | .882 | 46.5 ± 1.8 | 48.8 ± 1.9 | 49.0 ± 2.1 | .056 | .501 | .604 |
| MH | 49.3 ± 1.9 | 51.1 ± 2.4 | 52.1 ± 1.8 | .737 | .629 | .341 | 50.0 ± 2.1 | 51.4 ± 2.1 | 49.8 ± 1.9 | .987 | .464 | .908 |
| . | Male . | Female . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 49.9 ± 1.7 | 51.5 ± 1.5 | 52.9 ± 1.0 | .977 | .496 | .190 | 46.8 ± 2.7 | 48.5 ± 2.4 | 46.9 ± 2.8 | .082 | .422 | .094 |
| RP | 48.9 ± 2.9 | 49.1 ± 2.6 | 50.5 ± 2.1 | .620 | .672 | .819 | 45.1 ± 2.2 | 48.5 ± 1.8 | 46.1 ± 2.4 | .008 | .420 | .036 |
| BP | 49.7 ± 2.5 | 51.7 ± 2.5 | 53.2 ± 1.9 | .875 | .437 | .139 | 52.1 ± 2.2 | 50.2 ± 2.0 | 50.9 ± 2.1 | .247 | .923 | .633 |
| GH | 46.9 ± 1.4 | 47.8 ± 1.3 | 47.0 ± 1.1 | .159 | .322 | .166 | 47.3 ± 1.9 | 48.9 ± 1.6 | 47.9 ± 1.7 | .142 | .557 | .258 |
| VT | 50.0 ± 2.0 | 49.5 ± 1.8 | 50.9 ± 1.8 | .994 | .828 | .679 | 47.5 ± 2.0 | 49.3 ± 1.9 | 50.4 ± 1.6 | .179 | .697 | .836 |
| SF | 50.3 ± 2.1 | 52.1 ± 1.5 | 50.9 ± 1.8 | .902 | .334 | .686 | 46.6 ± 2.1 | 46.8 ± 2.2 | 46.6 ± 2.2 | .065 | .085 | .065 |
| RE | 48.1 ± 3.1 | 47.3 ± 3.1 | 50.3 ± 2.2 | .394 | .224 | .882 | 46.5 ± 1.8 | 48.8 ± 1.9 | 49.0 ± 2.1 | .056 | .501 | .604 |
| MH | 49.3 ± 1.9 | 51.1 ± 2.4 | 52.1 ± 1.8 | .737 | .629 | .341 | 50.0 ± 2.1 | 51.4 ± 2.1 | 49.8 ± 1.9 | .987 | .464 | .908 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50.
Abbreviations: PF, Physical Functioning; RP, Role-Physical; BP, Bodily Pain; GH, General Health; VT, Vitality; SF, Social Functioning; RE, Role-Emotional; MH, Mental Health.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in male and female PA patients compared with the general Japanese population
| . | Male . | Female . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 49.9 ± 1.7 | 51.5 ± 1.5 | 52.9 ± 1.0 | .977 | .496 | .190 | 46.8 ± 2.7 | 48.5 ± 2.4 | 46.9 ± 2.8 | .082 | .422 | .094 |
| RP | 48.9 ± 2.9 | 49.1 ± 2.6 | 50.5 ± 2.1 | .620 | .672 | .819 | 45.1 ± 2.2 | 48.5 ± 1.8 | 46.1 ± 2.4 | .008 | .420 | .036 |
| BP | 49.7 ± 2.5 | 51.7 ± 2.5 | 53.2 ± 1.9 | .875 | .437 | .139 | 52.1 ± 2.2 | 50.2 ± 2.0 | 50.9 ± 2.1 | .247 | .923 | .633 |
| GH | 46.9 ± 1.4 | 47.8 ± 1.3 | 47.0 ± 1.1 | .159 | .322 | .166 | 47.3 ± 1.9 | 48.9 ± 1.6 | 47.9 ± 1.7 | .142 | .557 | .258 |
| VT | 50.0 ± 2.0 | 49.5 ± 1.8 | 50.9 ± 1.8 | .994 | .828 | .679 | 47.5 ± 2.0 | 49.3 ± 1.9 | 50.4 ± 1.6 | .179 | .697 | .836 |
| SF | 50.3 ± 2.1 | 52.1 ± 1.5 | 50.9 ± 1.8 | .902 | .334 | .686 | 46.6 ± 2.1 | 46.8 ± 2.2 | 46.6 ± 2.2 | .065 | .085 | .065 |
| RE | 48.1 ± 3.1 | 47.3 ± 3.1 | 50.3 ± 2.2 | .394 | .224 | .882 | 46.5 ± 1.8 | 48.8 ± 1.9 | 49.0 ± 2.1 | .056 | .501 | .604 |
| MH | 49.3 ± 1.9 | 51.1 ± 2.4 | 52.1 ± 1.8 | .737 | .629 | .341 | 50.0 ± 2.1 | 51.4 ± 2.1 | 49.8 ± 1.9 | .987 | .464 | .908 |
| . | Male . | Female . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 49.9 ± 1.7 | 51.5 ± 1.5 | 52.9 ± 1.0 | .977 | .496 | .190 | 46.8 ± 2.7 | 48.5 ± 2.4 | 46.9 ± 2.8 | .082 | .422 | .094 |
| RP | 48.9 ± 2.9 | 49.1 ± 2.6 | 50.5 ± 2.1 | .620 | .672 | .819 | 45.1 ± 2.2 | 48.5 ± 1.8 | 46.1 ± 2.4 | .008 | .420 | .036 |
| BP | 49.7 ± 2.5 | 51.7 ± 2.5 | 53.2 ± 1.9 | .875 | .437 | .139 | 52.1 ± 2.2 | 50.2 ± 2.0 | 50.9 ± 2.1 | .247 | .923 | .633 |
| GH | 46.9 ± 1.4 | 47.8 ± 1.3 | 47.0 ± 1.1 | .159 | .322 | .166 | 47.3 ± 1.9 | 48.9 ± 1.6 | 47.9 ± 1.7 | .142 | .557 | .258 |
| VT | 50.0 ± 2.0 | 49.5 ± 1.8 | 50.9 ± 1.8 | .994 | .828 | .679 | 47.5 ± 2.0 | 49.3 ± 1.9 | 50.4 ± 1.6 | .179 | .697 | .836 |
| SF | 50.3 ± 2.1 | 52.1 ± 1.5 | 50.9 ± 1.8 | .902 | .334 | .686 | 46.6 ± 2.1 | 46.8 ± 2.2 | 46.6 ± 2.2 | .065 | .085 | .065 |
| RE | 48.1 ± 3.1 | 47.3 ± 3.1 | 50.3 ± 2.2 | .394 | .224 | .882 | 46.5 ± 1.8 | 48.8 ± 1.9 | 49.0 ± 2.1 | .056 | .501 | .604 |
| MH | 49.3 ± 1.9 | 51.1 ± 2.4 | 52.1 ± 1.8 | .737 | .629 | .341 | 50.0 ± 2.1 | 51.4 ± 2.1 | 49.8 ± 1.9 | .987 | .464 | .908 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50.
Abbreviations: PF, Physical Functioning; RP, Role-Physical; BP, Bodily Pain; GH, General Health; VT, Vitality; SF, Social Functioning; RE, Role-Emotional; MH, Mental Health.
Pre- and post-treatment SF-36 scores of male and female PA patients on an MRA. The SF-36 scores of male (A) and female (B) PA patients before treatment, and at 3 and 6 months after treatment, with MRAs. *P < .05 compared with the general Japanese population. Bar shows the SEM. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
Comparison of SF-36 Scores and Clinical Features Between Patients With Serum K Levels of <3.5 and ≥3.5 mEq/L
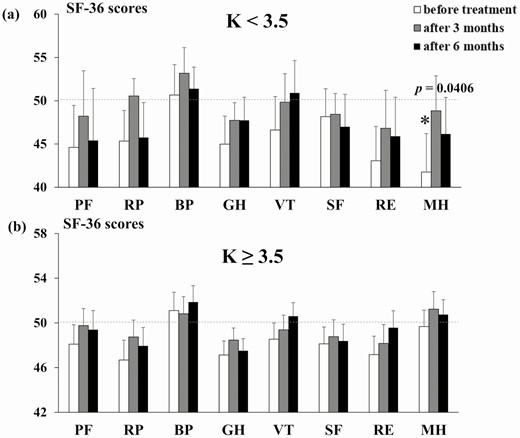
In total, 9 patients were in the serum K <3.5 mEq/L group and 41 were in the serum K ≥3.5 mEq/L group. The mean age, body mass index, and number of antihypertensive drugs excluding MRA of K <3.5 mEq/L group and K ≥3.5 mEq/L group were 57.1 ± 3.2 and 55.3 ± 1.9 years, 23.5 ± 1.1 and 21.9 ± 0.7 kg/m2, and 1.44 and 0.73, respectively. The SF-36 scores over time by MRA treatment are shown in Fig. 3 and Table 5; the clinical features are shown in Table 6. In the serum K <3.5 mEq/L group, the MH score was significantly lower than that of healthy subjects before treatment but was comparable to that of healthy subjects after treatment. There was a significant increase in K after MRA treatment, but no change in SBP, DBP, ARC, PAC, or ARR. In the K ≥3.5 mEq/L group, the total SF-36 score did not differ from that of healthy subjects, and a significant decrease in SBP and increase in K, ARC, and ARR were observed at 6 months after treatment compared to before treatment.
Clinical characteristics of hypokalemic and normokalemic PA patients, before and 3 and 6 months after, treatment with an MRA
| . | K < 3.5 . | K ≥ 3.5 . | K < 3.5 vs K ≥ 3.5 P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | Baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 9 . | . | . | . | . | 41 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 140.3 ± 7.0 | 127.1 ± 4.0 | .137 | 128.1 ± 1.4 | .199 | 140.7 ± 2.6 | 128.3 ± 2.2 | .009 | 124.5 ± 1.4 | <.001 | .955 | .809 | .270 |
| DBP (mmHg) | 84.6 ± 3.7 | 80.4 ± 2.5 | .577 | 79.7 ± 2.7 | .504 | 85.1 ± 2.2 | 82.3 ± 1.8 | .592 | 79.8 ± 1.7 | .146 | .931 | .601 | .982 |
| K (mEq/L) | 3.1 ± 0.2 | 4.2 ± 0.1 | .001 | 4.0 ± 0.2 | .002 | 4.0 ± 0.0 | 4.3 ± 0.1 | <.001 | 4.3 ± 0.1 | <.001 | <.001 | .408 | .073 |
| ARC (pg/mL) | 2.7 (2.0–5.0) | 3.8 (1.5–10.8) | .658 | 5.3 (3.3–9.0) | .517 | 2.2 (1.1–5.4) | 4.5 (2.7–10.4) | .088 | 5.1 (3.5–10.2) | .004 | .510 | .713 | .997 |
| PAC (pg/mL) | 205.8 (147.0–322.7) | 286.2 (207.6–315.0) | .860 | 286.4 (146.9–318.0) | .890 | 189.0 (150.3–227.7) | 203.5 (149.4–273.2) | .913 | 216.1 (135.7–280.5) | .309 | .261 | .099 | .417 |
| ARR (PAC/ARC) | 76.2 (52.6–433.5) | 44.6 (25.0–184.4) | .727 | 33.2 (20.8–119.7) | .359 | 63.6 (40.5–144.0) | 38.7 (19.8–76.0) | .146 | 33.0 (20.1–56.0) | .046 | .085 | .125 | .399 |
| MRA | |||||||||||||
| Spironolactone (N) | 1 | 1 | 13 | 12 | |||||||||
| Dose (mg) | 25.0 | 25.0 | 28.8 ± 2.6 | 29.2 ± 2.8 | |||||||||
| Eplerenone (N) | 7 | 7 | 23 | 23 | |||||||||
| Dose (mg) | 58.3 ± 0.0 | 58.3 ± 0.0 | 38.6 ± 2.7 | 40.9 ± 2.8 | |||||||||
| Esaxerenone (N) | 1 | 1 | 5 | 6 | |||||||||
| Dose (mg) | 2.5 | 2.5 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
| . | K < 3.5 . | K ≥ 3.5 . | K < 3.5 vs K ≥ 3.5 P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | Baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 9 . | . | . | . | . | 41 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 140.3 ± 7.0 | 127.1 ± 4.0 | .137 | 128.1 ± 1.4 | .199 | 140.7 ± 2.6 | 128.3 ± 2.2 | .009 | 124.5 ± 1.4 | <.001 | .955 | .809 | .270 |
| DBP (mmHg) | 84.6 ± 3.7 | 80.4 ± 2.5 | .577 | 79.7 ± 2.7 | .504 | 85.1 ± 2.2 | 82.3 ± 1.8 | .592 | 79.8 ± 1.7 | .146 | .931 | .601 | .982 |
| K (mEq/L) | 3.1 ± 0.2 | 4.2 ± 0.1 | .001 | 4.0 ± 0.2 | .002 | 4.0 ± 0.0 | 4.3 ± 0.1 | <.001 | 4.3 ± 0.1 | <.001 | <.001 | .408 | .073 |
| ARC (pg/mL) | 2.7 (2.0–5.0) | 3.8 (1.5–10.8) | .658 | 5.3 (3.3–9.0) | .517 | 2.2 (1.1–5.4) | 4.5 (2.7–10.4) | .088 | 5.1 (3.5–10.2) | .004 | .510 | .713 | .997 |
| PAC (pg/mL) | 205.8 (147.0–322.7) | 286.2 (207.6–315.0) | .860 | 286.4 (146.9–318.0) | .890 | 189.0 (150.3–227.7) | 203.5 (149.4–273.2) | .913 | 216.1 (135.7–280.5) | .309 | .261 | .099 | .417 |
| ARR (PAC/ARC) | 76.2 (52.6–433.5) | 44.6 (25.0–184.4) | .727 | 33.2 (20.8–119.7) | .359 | 63.6 (40.5–144.0) | 38.7 (19.8–76.0) | .146 | 33.0 (20.1–56.0) | .046 | .085 | .125 | .399 |
| MRA | |||||||||||||
| Spironolactone (N) | 1 | 1 | 13 | 12 | |||||||||
| Dose (mg) | 25.0 | 25.0 | 28.8 ± 2.6 | 29.2 ± 2.8 | |||||||||
| Eplerenone (N) | 7 | 7 | 23 | 23 | |||||||||
| Dose (mg) | 58.3 ± 0.0 | 58.3 ± 0.0 | 38.6 ± 2.7 | 40.9 ± 2.8 | |||||||||
| Esaxerenone (N) | 1 | 1 | 5 | 6 | |||||||||
| Dose (mg) | 2.5 | 2.5 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
Data of PAC, ARC, and ARR are expressed as the median (25th percentile-75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
aP values for comparison of K < 3.5 data at baseline.
bP values for comparison of K ≥ 3.5 data at baseline.
Clinical characteristics of hypokalemic and normokalemic PA patients, before and 3 and 6 months after, treatment with an MRA
| . | K < 3.5 . | K ≥ 3.5 . | K < 3.5 vs K ≥ 3.5 P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | Baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 9 . | . | . | . | . | 41 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 140.3 ± 7.0 | 127.1 ± 4.0 | .137 | 128.1 ± 1.4 | .199 | 140.7 ± 2.6 | 128.3 ± 2.2 | .009 | 124.5 ± 1.4 | <.001 | .955 | .809 | .270 |
| DBP (mmHg) | 84.6 ± 3.7 | 80.4 ± 2.5 | .577 | 79.7 ± 2.7 | .504 | 85.1 ± 2.2 | 82.3 ± 1.8 | .592 | 79.8 ± 1.7 | .146 | .931 | .601 | .982 |
| K (mEq/L) | 3.1 ± 0.2 | 4.2 ± 0.1 | .001 | 4.0 ± 0.2 | .002 | 4.0 ± 0.0 | 4.3 ± 0.1 | <.001 | 4.3 ± 0.1 | <.001 | <.001 | .408 | .073 |
| ARC (pg/mL) | 2.7 (2.0–5.0) | 3.8 (1.5–10.8) | .658 | 5.3 (3.3–9.0) | .517 | 2.2 (1.1–5.4) | 4.5 (2.7–10.4) | .088 | 5.1 (3.5–10.2) | .004 | .510 | .713 | .997 |
| PAC (pg/mL) | 205.8 (147.0–322.7) | 286.2 (207.6–315.0) | .860 | 286.4 (146.9–318.0) | .890 | 189.0 (150.3–227.7) | 203.5 (149.4–273.2) | .913 | 216.1 (135.7–280.5) | .309 | .261 | .099 | .417 |
| ARR (PAC/ARC) | 76.2 (52.6–433.5) | 44.6 (25.0–184.4) | .727 | 33.2 (20.8–119.7) | .359 | 63.6 (40.5–144.0) | 38.7 (19.8–76.0) | .146 | 33.0 (20.1–56.0) | .046 | .085 | .125 | .399 |
| MRA | |||||||||||||
| Spironolactone (N) | 1 | 1 | 13 | 12 | |||||||||
| Dose (mg) | 25.0 | 25.0 | 28.8 ± 2.6 | 29.2 ± 2.8 | |||||||||
| Eplerenone (N) | 7 | 7 | 23 | 23 | |||||||||
| Dose (mg) | 58.3 ± 0.0 | 58.3 ± 0.0 | 38.6 ± 2.7 | 40.9 ± 2.8 | |||||||||
| Esaxerenone (N) | 1 | 1 | 5 | 6 | |||||||||
| Dose (mg) | 2.5 | 2.5 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
| . | K < 3.5 . | K ≥ 3.5 . | K < 3.5 vs K ≥ 3.5 P value . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | 3 months . | P valuea . | 6 months . | P valuea . | Baseline . | 3 months . | P valueb . | 6 months . | P valueb . | Baseline . | 3 months . | 6 months . |
| N . | 9 . | . | . | . | . | 41 . | . | . | . | . | . | . | . |
| SBP (mmHg) | 140.3 ± 7.0 | 127.1 ± 4.0 | .137 | 128.1 ± 1.4 | .199 | 140.7 ± 2.6 | 128.3 ± 2.2 | .009 | 124.5 ± 1.4 | <.001 | .955 | .809 | .270 |
| DBP (mmHg) | 84.6 ± 3.7 | 80.4 ± 2.5 | .577 | 79.7 ± 2.7 | .504 | 85.1 ± 2.2 | 82.3 ± 1.8 | .592 | 79.8 ± 1.7 | .146 | .931 | .601 | .982 |
| K (mEq/L) | 3.1 ± 0.2 | 4.2 ± 0.1 | .001 | 4.0 ± 0.2 | .002 | 4.0 ± 0.0 | 4.3 ± 0.1 | <.001 | 4.3 ± 0.1 | <.001 | <.001 | .408 | .073 |
| ARC (pg/mL) | 2.7 (2.0–5.0) | 3.8 (1.5–10.8) | .658 | 5.3 (3.3–9.0) | .517 | 2.2 (1.1–5.4) | 4.5 (2.7–10.4) | .088 | 5.1 (3.5–10.2) | .004 | .510 | .713 | .997 |
| PAC (pg/mL) | 205.8 (147.0–322.7) | 286.2 (207.6–315.0) | .860 | 286.4 (146.9–318.0) | .890 | 189.0 (150.3–227.7) | 203.5 (149.4–273.2) | .913 | 216.1 (135.7–280.5) | .309 | .261 | .099 | .417 |
| ARR (PAC/ARC) | 76.2 (52.6–433.5) | 44.6 (25.0–184.4) | .727 | 33.2 (20.8–119.7) | .359 | 63.6 (40.5–144.0) | 38.7 (19.8–76.0) | .146 | 33.0 (20.1–56.0) | .046 | .085 | .125 | .399 |
| MRA | |||||||||||||
| Spironolactone (N) | 1 | 1 | 13 | 12 | |||||||||
| Dose (mg) | 25.0 | 25.0 | 28.8 ± 2.6 | 29.2 ± 2.8 | |||||||||
| Eplerenone (N) | 7 | 7 | 23 | 23 | |||||||||
| Dose (mg) | 58.3 ± 0.0 | 58.3 ± 0.0 | 38.6 ± 2.7 | 40.9 ± 2.8 | |||||||||
| Esaxerenone (N) | 1 | 1 | 5 | 6 | |||||||||
| Dose (mg) | 2.5 | 2.5 | 2.5 ± 0.0 | 2.5 ± 0.0 | |||||||||
Data of PAC, ARC, and ARR are expressed as the median (25th percentile-75th percentile), and the other data are expressed as the mean ± standard error of the mean (SEM).
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; ARC, active renin concentration; PAC, plasma aldosterone concentration; ARR, aldosterone renin ratio; MRA, mineralocorticoid receptor antagonist.
aP values for comparison of K < 3.5 data at baseline.
bP values for comparison of K ≥ 3.5 data at baseline.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in hypokalemic and normokalemic PA patients compared with the general Japanese population
| . | . | . | . | K < 3.5 . | . | . | K ≥ 3.5 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | Baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | Baseline . | 3 months . | 6 months . | Baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 44.6 ± 4.9 | 48.2 ± 5.2 | 45.4 ± 6.0 | .106 | .593 | .169 | 48.9 ± 1.8 | 50.1 ± 1.5 | 50.3 ± 1.6 | .467 | .951 | .861 |
| RP | 46.5 ± 3.5 | 50.6 ± 2.0 | 45.8 ± 4.0 | .292 | .869 | .202 | 46.7 ± 2.0 | 48.3 ± 1.8 | 48.4 ± 1.8 | .036 | .289 | .313 |
| BP | 51.9 ± 3.5 | 53.2 ± 3.0 | 51.4 ± 2.5 | .571 | .340 | .676 | 50.9 ± 1.4 | 50.3 ± 1.8 | 52.0 ± 1.7 | .552 | .849 | .206 |
| GH | 46.7 ± 3.2 | 47.7 ± 2.0 | 47.7 ± 2.7 | .318 | .498 | .498 | 47.2 ± 1.4 | 48.6 ± 1.3 | 47.5 ± 1.2 | .076 | .376 | .104 |
| VT | 47.7 ± 3.9 | 49.8 ± 3.3 | 50.9 ± 3.7 | .488 | .960 | .787 | 48.7 ± 1.6 | 49.3 ± 1.5 | 50.5 ± 1.2 | .418 | .646 | .731 |
| SF | 48.4 ± 3.2 | 48.4 ± 2.4 | 47.0 ± 3.7 | .637 | .637 | .368 | 48.1 ± 1.7 | 49.2 ± 1.7 | 48.7 ± 1.7 | .215 | .591 | .402 |
| RE | 44.5 ± 4.0 | 46.8 ± 4.4 | 45.9 ± 4.5 | .099 | .340 | .218 | 47.7 ± 1.8 | 48.5 ± 1.8 | 50.4 ± 1.6 | .149 | .323 | .805 |
| MH | 43.2 ± 4.4 | 48.8 ± 4.0 | 46.2 ± 4.2 | .041 | .728 | .249 | 51.1 ± 1.4 | 51.8 ± 1.7 | 51.8 ± 1.3 | .481 | .261 | .261 |
| . | . | . | . | K < 3.5 . | . | . | K ≥ 3.5 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | Baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | Baseline . | 3 months . | 6 months . | Baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 44.6 ± 4.9 | 48.2 ± 5.2 | 45.4 ± 6.0 | .106 | .593 | .169 | 48.9 ± 1.8 | 50.1 ± 1.5 | 50.3 ± 1.6 | .467 | .951 | .861 |
| RP | 46.5 ± 3.5 | 50.6 ± 2.0 | 45.8 ± 4.0 | .292 | .869 | .202 | 46.7 ± 2.0 | 48.3 ± 1.8 | 48.4 ± 1.8 | .036 | .289 | .313 |
| BP | 51.9 ± 3.5 | 53.2 ± 3.0 | 51.4 ± 2.5 | .571 | .340 | .676 | 50.9 ± 1.4 | 50.3 ± 1.8 | 52.0 ± 1.7 | .552 | .849 | .206 |
| GH | 46.7 ± 3.2 | 47.7 ± 2.0 | 47.7 ± 2.7 | .318 | .498 | .498 | 47.2 ± 1.4 | 48.6 ± 1.3 | 47.5 ± 1.2 | .076 | .376 | .104 |
| VT | 47.7 ± 3.9 | 49.8 ± 3.3 | 50.9 ± 3.7 | .488 | .960 | .787 | 48.7 ± 1.6 | 49.3 ± 1.5 | 50.5 ± 1.2 | .418 | .646 | .731 |
| SF | 48.4 ± 3.2 | 48.4 ± 2.4 | 47.0 ± 3.7 | .637 | .637 | .368 | 48.1 ± 1.7 | 49.2 ± 1.7 | 48.7 ± 1.7 | .215 | .591 | .402 |
| RE | 44.5 ± 4.0 | 46.8 ± 4.4 | 45.9 ± 4.5 | .099 | .340 | .218 | 47.7 ± 1.8 | 48.5 ± 1.8 | 50.4 ± 1.6 | .149 | .323 | .805 |
| MH | 43.2 ± 4.4 | 48.8 ± 4.0 | 46.2 ± 4.2 | .041 | .728 | .249 | 51.1 ± 1.4 | 51.8 ± 1.7 | 51.8 ± 1.3 | .481 | .261 | .261 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
SF-36 scores at baseline and 3 and 6 months after treatment with MRAs in hypokalemic and normokalemic PA patients compared with the general Japanese population
| . | . | . | . | K < 3.5 . | . | . | K ≥ 3.5 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | Baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | Baseline . | 3 months . | 6 months . | Baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 44.6 ± 4.9 | 48.2 ± 5.2 | 45.4 ± 6.0 | .106 | .593 | .169 | 48.9 ± 1.8 | 50.1 ± 1.5 | 50.3 ± 1.6 | .467 | .951 | .861 |
| RP | 46.5 ± 3.5 | 50.6 ± 2.0 | 45.8 ± 4.0 | .292 | .869 | .202 | 46.7 ± 2.0 | 48.3 ± 1.8 | 48.4 ± 1.8 | .036 | .289 | .313 |
| BP | 51.9 ± 3.5 | 53.2 ± 3.0 | 51.4 ± 2.5 | .571 | .340 | .676 | 50.9 ± 1.4 | 50.3 ± 1.8 | 52.0 ± 1.7 | .552 | .849 | .206 |
| GH | 46.7 ± 3.2 | 47.7 ± 2.0 | 47.7 ± 2.7 | .318 | .498 | .498 | 47.2 ± 1.4 | 48.6 ± 1.3 | 47.5 ± 1.2 | .076 | .376 | .104 |
| VT | 47.7 ± 3.9 | 49.8 ± 3.3 | 50.9 ± 3.7 | .488 | .960 | .787 | 48.7 ± 1.6 | 49.3 ± 1.5 | 50.5 ± 1.2 | .418 | .646 | .731 |
| SF | 48.4 ± 3.2 | 48.4 ± 2.4 | 47.0 ± 3.7 | .637 | .637 | .368 | 48.1 ± 1.7 | 49.2 ± 1.7 | 48.7 ± 1.7 | .215 | .591 | .402 |
| RE | 44.5 ± 4.0 | 46.8 ± 4.4 | 45.9 ± 4.5 | .099 | .340 | .218 | 47.7 ± 1.8 | 48.5 ± 1.8 | 50.4 ± 1.6 | .149 | .323 | .805 |
| MH | 43.2 ± 4.4 | 48.8 ± 4.0 | 46.2 ± 4.2 | .041 | .728 | .249 | 51.1 ± 1.4 | 51.8 ± 1.7 | 51.8 ± 1.3 | .481 | .261 | .261 |
| . | . | . | . | K < 3.5 . | . | . | K ≥ 3.5 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | SF-36 scores . | P values . | SF-36 scores . | P values . | ||||||||
| . | Baseline . | 3 months . | 6 months . | baseline vs general population . | 3 months vs general population . | 6 months vs general population . | Baseline . | 3 months . | 6 months . | Baseline vs general population . | 3 months vs general population . | 6 months vs general population . |
| PF | 44.6 ± 4.9 | 48.2 ± 5.2 | 45.4 ± 6.0 | .106 | .593 | .169 | 48.9 ± 1.8 | 50.1 ± 1.5 | 50.3 ± 1.6 | .467 | .951 | .861 |
| RP | 46.5 ± 3.5 | 50.6 ± 2.0 | 45.8 ± 4.0 | .292 | .869 | .202 | 46.7 ± 2.0 | 48.3 ± 1.8 | 48.4 ± 1.8 | .036 | .289 | .313 |
| BP | 51.9 ± 3.5 | 53.2 ± 3.0 | 51.4 ± 2.5 | .571 | .340 | .676 | 50.9 ± 1.4 | 50.3 ± 1.8 | 52.0 ± 1.7 | .552 | .849 | .206 |
| GH | 46.7 ± 3.2 | 47.7 ± 2.0 | 47.7 ± 2.7 | .318 | .498 | .498 | 47.2 ± 1.4 | 48.6 ± 1.3 | 47.5 ± 1.2 | .076 | .376 | .104 |
| VT | 47.7 ± 3.9 | 49.8 ± 3.3 | 50.9 ± 3.7 | .488 | .960 | .787 | 48.7 ± 1.6 | 49.3 ± 1.5 | 50.5 ± 1.2 | .418 | .646 | .731 |
| SF | 48.4 ± 3.2 | 48.4 ± 2.4 | 47.0 ± 3.7 | .637 | .637 | .368 | 48.1 ± 1.7 | 49.2 ± 1.7 | 48.7 ± 1.7 | .215 | .591 | .402 |
| RE | 44.5 ± 4.0 | 46.8 ± 4.4 | 45.9 ± 4.5 | .099 | .340 | .218 | 47.7 ± 1.8 | 48.5 ± 1.8 | 50.4 ± 1.6 | .149 | .323 | .805 |
| MH | 43.2 ± 4.4 | 48.8 ± 4.0 | 46.2 ± 4.2 | .041 | .728 | .249 | 51.1 ± 1.4 | 51.8 ± 1.7 | 51.8 ± 1.3 | .481 | .261 | .261 |
SF-36 scores are expressed as the mean ± standard error of the mean (SEM). The mean value of general Japanese population is taken as 50. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
Pre- and post-treatment SF-36 scores of hypokalemic and normokalemic PA patients. The SF-36 scores of the serum K <3.5 mEq/L (A) and ≥3.5 mEq/L (B) PA patient groups before treatment, and at 3 and 6 months after treatment, with MRAs. *P < .05 compared with the general Japanese population. Bar shows the SEM. Abbreviations: BP, Bodily Pain; GH, General Health; MH, Mental Health; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
Discussion
This study showed for the first time that treatment with an MRA improves QOL in Japanese PA patients. The female patients showed a smaller increase in ARC and no improvement in QOL after treatment with an MRA. Furthermore, in patients with serum K levels <3.5 mEq/L, psychological problems might be improved by MRAs.
This study is the first to report improved QOL after administration of an MRA in Japanese PA patients. The scores for the GH and RE, which are psychological SF-36 domains, and the RP, which is a physical domain, were lower in patients with PA before treatment than in healthy subjects. MRAs improved the GH, RE, and RP scores in the patients, such that they were similar to those of healthy subjects. In previous reports using the SF-36, Ahmed et al. [9] reported a decrease in PF, RP, GH, and MH; Velema et al. [12] reported a decrease in PF, RP, GH, VT, SF, RE, and MH before treatment; and Ishidoya et al. [14] reported that only GH was decreased in Japanese PA patients before adrenalectomy. Thus, in these reports, the mental and physical QOL of PA patients were impaired, consistent with the RE, RP, and GH domain impairments seen in this study. When aldosterone is administered to rats, they exhibit anxious behavior [19], which is reduced by administration of MRAs [20]. In humans, angiotensin-converting enzyme inhibitors and angiotensin receptor blocker improve anxiety [21, 22], suggesting that MR overactivation is associated with anxious behavior. PA is often comorbid with obstructive sleep apnea syndrome (OSAS), and it is thought physical QOL may be affected by OSAS [23]. MRAs improve OSAS [24], which may explain the improved physical QOL seen in the PA patients in this study, assuming that some of them also suffered from OSAS.
The RP score of the female PA patients in this study was lower than that of the males and was not improved by MRA treatment. In previous studies, female PA patients had lower mental and physical QOL than males [11, 18, 25]. In this study, the psychological SF-36 domain scores were not different between healthy subjects and male or female PA patients, although the RP score was lower in female PA patients. The lower QOL of our female PA patients was consistent with previous reports [11, 18, 25], which postulated that female patients may experience more side effects from MRA treatment [11]; females also have higher aldosterone levels and are more likely to suffer from hyperaldosteronism [11, 25]. Patients were treated with eplerenone [26] and esaxerenone [27] in previous studies, which have high selectivity for MR; in this study, more patients were treated with these agents than with spironolactone. Lower SF-36 scores were not found to be associated with the use of spironolactone. The aldosterone concentration was not different between the males and females in this study, in contrast to previous reports in which females had higher levels. The ARC was significantly increased in males after treatment, but not in females. This was a novel finding; previous studies did not investigate changes in hormones or QOL according to both MRA treatment and sex. In this study, the MRA treatment was associated with improved BP control, and serum K and renin levels. In a previous study, compared with PA and essential hypertensive patients, PA patients with a PRA level >1 ng/mL/hour after treatment showed significantly better cardiovascular outcomes than those with PRA <1 ng/mL/hour, as well as essential hypertensive patients [28]. According to the Japanese Society of Hypertension Guidelines for the Management of Hypertension 2019, the ARC value is about 5-fold of the PRA value [6]. In this study, our post-treatment ARC target was ARC >5 pg/mL. In all patients and male patients with improved QOL, ARC was significantly elevated after treatment with MRAs with the median ARC was higher than 5 pg/mL (5.1 and 7.4, respectively). In contrast, the female patients who did not show improvement in QOL showed a nonsignificant increase in ARC after treatment with MRAs, and the median ARC was 4.1 pg/mL. Although it is not possible to determine whether ARC >5 pg/mL is an indicator of improved QOL in this study, it may be better to treat with MRA to achieve this target. The minimal increase in ARC in our female patients suggests that MRAs may not be able to suppress MR activation to a sufficient degree; alternatively, the salt intake of our patients may have been too high for ARC suppression, and hypertensive patients with genetically low renin levels may have been included. Regarding the first possibility, female patients may have had a lower ARC because they were more likely to be given eplerenone more than the males, where eplerenone has a lower affinity for MR than spironolactone and esaxerenone [29]. The other possibilities mentioned above were not investigated.
No previous reports evaluated the QOL of PA patients before the start of treatment according to K levels. Our PA group with a serum K level <3.5 mEq/L had a lower MH than the healthy subjects, although this was improved by MRA treatment such that the levels were similar between the 2 populations. However, there were only 9 patients with hypokalemia, and the improvement of QOL by MRAs was very preliminary. Hypokalemia is a well-known marker of MR activation in the kidney. The significantly lower MH score of the serum K <3.5 mEq/L group was likely the result of MR overactivation.
PA treatment should be delivered in accordance with AVS results [30]. However, in some cases AVS cannot be performed, due to patient refusal, poor general condition, or allergies. The results of this study suggested that the QOL of PA patients with hypokalemia not undergoing AVS may be improved by MRAs. The QOL scores that had decreased before treatment remained within the normal range at 6 months after MRA treatment in the serum K <3.5 mEq/L group. MRAs improved clinical feature such as SBP, serum K, ARC, and ARR in the serum K ≥3.5 mEq/L group, whereas only the serum K was improved in the K <3.5 mEq/L group. This may be due to the small number of included cases, or to the hypokalemia patients being resistant to improvements in SBP, DBP, ARC, and ARR. The improvement in MH (despite the lack of improvement in ARC) may not be related to MR activation.
This study had some limitations. First, the follow-up period was only 6 months, so long-term outcomes are unknown. Second, the degree of improvement in SAS after treatment may have an effect on the decrease in physical QOL, but SAS has not been evaluated. Third, it remains unclear whether MRAs are effective against unilateral PA. Fourth, the female PA patients showed little increase in ARC even after the start of MRA treatment compared to males, but salt intake could not be evaluated; this is important because ARC is reduced by excessive salt intake. Sixth, the questionnaire may have influenced the results in this study, but it could not be evaluated because there was no control group.
Conclusions
For the first time, this study showed that MRAs improve QOL in Asian patients with PA. The female patients showed less improvement in QOL than the males and were less likely to show an increase in the ARC. In addition, PA patients with hypokalemia who had a serum K level <3.5 mEq/L showed a particularly low psychological QOL, which might be improved by MRAs.
Abbreviations
- ARC
active renin concentration
- ARR
ARC ratio
- AVS
adrenal vein sampling
- BP
Bodily Pain
- DBP
diastolic blood pressure
- GH
General Health
- MH
Mental Health
- MR
mineralocorticoid receptor
- MRA
mineralocorticoid receptor antagonist
- OSAS
obstructive sleep apnea syndrome
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PF
Physical Functioning
- RE
Role-Emotional
- RP
Role-Physical
- SBP
systolic blood pressure
- SEM
standard error of the mean
- SF
Social Functioning
- SF-36
36-Item Short-Form Health Survey
- QOL
quality of life
- VT
Vitality
Acknowledgments
We thank the doctors, medical staff, and patients who answered the questionnaire for their cooperation in this study.
Clinical Trial Information: Clinical Trial Registration Number: 909 (registered June 12, 2017) and 1761 (registered January 20, 2020).
Additional Information
Disclosures: The authors have nothing to disclose
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.