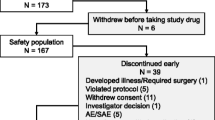
Abstract
Migraine headaches are usually intolerable, and a quick-relief treatment remains an unmet medical need. Almotriptan malate is a serotonin (5-HT1B/1D) receptor agonist approved for the treatment of acute migraine in adults. It is currently available in an oral tablet dosage form and has a Tmax of 1–3 h, and therefore, there is a medical need to develop a non-invasive rapidly acting formulation. We have developed an intranasal formulation of almotriptan malate using the quality-by-design (QbD) approach. A 2-factor 3-level full factorial design was selected to build up the experimental setting. The developed formulation was characterized for pH, viscosity, in vitro permeation, ex vivo permeation, and histopathological tolerance. To assess the potential of the developed formulation to produce a rapid onset of action following intranasal delivery, a pharmacokinetic study was performed in the Sprague–Dawley rat model and compared to the currently available marketed oral tablet formulation. For this, the LC–MS/MS bioanalytical method was developed and used for the determination of plasma almotriptan malate concentrations. Results of a pharmacokinetic study revealed that intranasal administration of optimized almotriptan malate formulation enabled an almost five-fold reduction in Tmax and about seven-fold increase in bioavailability in comparison to the currently available oral tablet formulation, suggesting the potential of developed almotriptan malate intranasal formulation in producing a rapid onset of action as well as enhanced bioavailability.
Graphical Abstract








Similar content being viewed by others
Data Availability
All data underlying the results are available as part of the article and no additional source data are required.
References
Goadsby PJ, Lipton RB, Ferrari MD. Migraine — current understanding and treatment. N Engl J Med. 2002Jan 24;346(4):257–70.
Girotra P, Singh SK. Multivariate optimization of rizatriptan benzoate-loaded solid lipid nanoparticles for brain targeting and migraine management. AAPS PharmSciTech. 2017Feb 1;18(2):517–28.
Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, et al. Migraine: epidemiology and systems of care. Lancet. 2021Apr 17;397(10283):1485–95.
Cameron C, Kelly S, Hsieh SC, Murphy M, Chen L, Kotb A, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(Suppl 4(S4)):221–35.
Dahlöf CGH, Dodick D, Dowson AJ, Pascual J. How does almotriptan compare with other triptans? A review of data from placebo-controlled clinical trials. Headache. 2002;42(2):99–113.
Balbisi EA. Efficacy and safety of almotriptan malate for migraine. Am J Health Syst Pharm. 2002Nov 15;59(22):2184–93.
Olesen A, Schytz HW, Ostrowski SR, Topholm M, Nielsen K, Erikstrup C, et al. Low adherence to the guideline for the acute treatment of migraine. Sci Rep. 2022;12:8487.
Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache J Head Face Pain. 1999;39(SUPPL. 2):S20–6.
Ashish G, Mahesh M, Sharma Pravin K, Ravi S, Koka Sweta S, Gajanan D. Formulation and evaluation of mouth dissolving tablet of almotriptan malate. Am J PharmTech Res. 2021;11(05):63–9.
Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Saraiya V, Attimarad M, et al. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020Feb 1;28(2):201–9.
Calatayud-Pascual MA, Balaguer-Fernández C, Serna-Jiménez CE, Del Rio-Sancho S, Femenía-Font A, Merino V, et al. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharm. 2011Sep 15;416(1):189–94.
Salave S, Rana D, Pardhe R, Bule P, Benival D. Unravelling micro and nano vesicular system in intranasal drug delivery for epilepsy. Pharm Nanotechnol. 2022Apr 27;10(3):182–93.
Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2010Feb 18;53(1):3–21.
Brackenridge A, Wallbank H, Lawrenson RA, Russell-Jones D. Emergency management of diabetes and hypoglycaemia. Emerg Med J. 2006Mar 1;23(3):183–5.
Caputo N, Castle JR, Bergstrom CP, Carroll JM, Bakhtiani PA, Jackson MA, et al. Mechanisms of glucagon degradation at alkaline pH. Peptides. 2013Jul;1(45):40–7.
FDA, CDER. BAQSIMI (glucagon) nasal powder. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf. Accessed 21 Sep 2022.
Suico JG, Hövelmann U, Zhang S, Shen T, Bergman B, Sherr J, et al. Glucagon administration by nasal and intramuscular routes in adults with type 1 diabetes during insulin-induced hypoglycaemia: a randomised, open-label, crossover study. Diabetes Ther. 2020Jul 1;11(7):1591–603.
FDA. NARCAN (naloxone hydrochloride) nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208411lbl.pdf. Accessed 21 Sep 2022.
ARS Pharmaceuticals, Inc. to Share New Data at the 2022 American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting. 2002. https://www.prnewswire.com/news-releases/ars-pharmaceuticals-inc-to-share-new-data-at-the-2022-american-academy-of-allergy-asthma--immunology-aaaai-annual-meeting-301473358.html. Accessed 21 Sep 2022.
Gao M, Shen X, Mao S. Factors influencing drug deposition in the nasal cavity upon delivery via nasal sprays. J Pharm Investig. 2020May 1;50(3):251–9.
Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002Dec 1;7(23):1184–9.
Pradhan V, Gaikwad R, Samad A, Prabhakar B. Formulation and evaluation of almotriptan malate nasal drops. Indian J Pharm Sci. 2009;71(6):727.
Pailla SR, Talluri S, Rangaraj N, Ramavath R, Challa VS, Doijad N, et al. Intranasal zotepine nanosuspension: intended for improved brain distribution in rats. DARU J Pharm Sci. 2019Jun 29;27(2):541–56.
Bartos C, Pallagi E, Szabó-Révész P, Ambrus R, Katona G, Kiss T, et al. Formulation of levodopa containing dry powder for nasal delivery applying the quality-by-design approach. Eur J Pharm Sci. 2018Oct;15(123):475–83.
Dua R, Zia H, Needham T. The influence of tonicity and viscosity on the intranasal absorption of salmon calcitonin in rabbits. Int J Pharm. 1997Feb 28;147(2):233–42.
Static cell (vertical or Side-Bi-Side). PermeGear. https://permegear.com/. Accessed 23 Nov 2022.
H&E staining kit (hematoxylin and eosin) (ab245880). Abcam. https://www.abcam.com/he-staining-kit-hematoxylin-and-eosin-ab245880.html. Accessed 21 Sep 2022.
Williams AJ, Jordan F, King G, Lewis AL, Illum L, Masud T, et al. In vitro and preclinical assessment of an intranasal spray formulation of parathyroid hormone PTH 1–34 for the treatment of osteoporosis. Int J Pharm. 2018Jan 15;535(1–2):113–9.
Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27.
Tan A, Awaiye K. Use of internal standards in LC-MS bioanalysis. In: Li W, Zhang J Tse FLS, editors. Handbook of LC-MS bioanalysis: best practices, experimental protocols, and regulations. New Jersey: Wiley; 2013. p. 217–27.
Rathod M, Suthar D, Patel H, Shelat P, Parejiya P. Microemulsion based nasal spray: a systemic approach for non-CNS drug, its optimization, characterization and statistical modelling using QbD principles. J Drug Deliv Sci Technol. 2019Feb;1(49):286–300.
Salade L, Wauthoz N, Goole J, Amighi K. How to characterize a nasal product. The state of the art of in vitro and ex vivo specific methods. Int J Pharm. 2019;561:47–65.
Youssef NAHA, Kassem AA, Farid RM, Ismail FA, EL-Massik MAE Boraie NA. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: preparation, characterization and in vivo evaluation. Int J Pharm. 2018;548(1):609–24.
Salem LH, El-Feky GS, Fahmy RH, El Gazayerly ON, Abdelbary A. Coated lipidic nanoparticles as a new strategy for enhancing nose-to-brain delivery of a hydrophilic drug molecule. J Pharm Sci. 2020Jul 1;109(7):2237–51.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shubham Gupta: investigation, methodology, visualization, writing original draft.
Akhil Perla: investigation, methodology, visualization, writing original draft.
Abhishek Roy: investigation, methodology.
Jyotsna G. Vitore: writing, reviewing, and editing.
Bharathi K: reviewing, and editing.
Sagar Salave: writing, data curation, reviewing, and editing.
Dhwani Rana: reviewing, and editing.
Amit Sharma: reviewing, and editing.
Rajeshwari Rathod: investigation, reviewing.
Hemant Kumar: investigation, reviewing, visualization.
Derajram Benival: conceptualization, methodology, visualization, supervision, and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study employed Sprague–Dawley rats which were treated according to standard laboratory guidelines according to a protocol approved by the Institutional Animal Ethics Committee of NIPER-A (NIPERA/IAEC/2020/031).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, S., Perla, A., Roy, A. et al. In Vivo Evaluation of Almotriptan malate Formulation through Intranasal Route for the Treatment of Migraine: Systematic Development and Pharmacokinetic Assessment. AAPS PharmSciTech 24, 32 (2023). https://doi.org/10.1208/s12249-022-02496-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02496-2