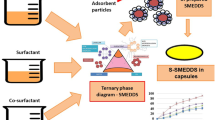
Abstract
Efavirenz (EFV) is an anti-HIV drug with high dose and 40% oral bioavailability (BA). The aim was to improve the bioavailability by designing cationic solid SMEDDS. Solubility data, ternary phase diagrams, and central composite design were employed in design. Globule size, TEM, DSC, and SEM studies were used for characterization. Optimized L-SMEDDS contained 20 mg of EFV, 10 mg of Peceol, 43.5 mg of Tween 80, and 40 mg of Labrafac Lipophile WL-1349 and the characters included mean globule size-94 nm, PDI-0.255, and ZP-28 mV. Later, octadecylamine was added to get L-SMEDDS with + 38 mV charge. L-SMEDDS was converted into solid S-SMEDDS by adsorbing onto silica carriers. Syloid XDP was preferred based on flow and oil adsorption capacity. The % drug (EFV) release from powder, L-SMEDDS, and solid SMEDDS were 14.04, 94.47, and 85 respectively in first 30 min. TEM picture showed dispersed globules. DSC and SEM studies indicated the loss of drug crystallinity in S-SMEDDS. Pharmacokinetic (PK) studies in Wistar rats revealed 4.12 fold hike in BA for optimized cationic S-SMEDDS when compared to EFV suspension. Increased absorption could be due to the positive charge on globules. Thus, cationic S-SMEDDS emerged as a potential novel delivery system for improvement in BA and has scope for reducing the high dose for AIDS patients by future clinical studies.
Graphical Abstract











Similar content being viewed by others
References
Avachat AM, Parpani SS. Formulation and development of bicontinuous nanostructured liquid crystalline particles of efavirenz. Colloids Surf B. 2015;126:87–97.
Deshmukh A, Kulakrni S. Novel self micro-emulsifying drug delivery systems (SMEDDS) of efavirenz. J Chem Pharm Res. 2012;4:3914–9.
Deshmukh A, Kulkarni S. Solid self-microemulsifying drug delivery system of ritonavir. Drug Dev Ind Pharm. 2014;40(4):477–87.
Kamble RN, Mehta PP, Kumar A. Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS PharmSciTech. 2016;17(5):1240–7.
Okafor NI, Nkanga CI, Walker RB, Noundou XS, Krause RWM. Encapsulation and physicochemical evaluation of efavirenz in liposomes. J Pharm Investig. 2020;50(2):201–8.
Rao MR, Babrekar LS. Liposomal drug delivery for solubility and bioavailability enhancement of efavirenz. Indian J Pharm Sci. 2018;80(6):1115–24.
Pokharkar V, Patil-Gadhe A, Palla P. Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: in-vivo pharmacokinetic and toxicity study. Biomed Pharmacother. 2017;94:150–64.
Lavra ZMM, Pereira de Santana D, Ré MI. Solubility and dissolution performances of spray-dried solid dispersion of efavirenz in Soluplus. Drug Dev Ind Pharm. 2017;43(1):42–54.
Prakash CS, Sunit KS, Alakh NS. Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed Pharmacother. 2016;80:42–51.
Chandrakant K, Dhanashri K, Dnyandev G, Chandrashekar M, Gajendra K. Efavirenz-loaded intranasal microemulsion for crossing blood-CNS interfaces in neuronal-AIDS: pharmacokinetic and in vivo safety evaluation. Pharm Dev Technol. 2020;25(1):28–39.
Choo GH, Park SJ, Hwang SJ, Kim MS. Formulation and in vivo evaluation of a self-microemulsifying drug delivery system of dutasteride. Drug Res. 2013;63(4):203–9.
Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15(21–22):958–65.
Khaled MH, Hibah MA, Rahaf HB, Amal MS, Mallesh K, Majed MA, Ahmed YA, Hala MA, Hiba HB, Rasha AK, Amani ME. Preparation, optimization, and evaluation of hyaluronic acid-based hydrogel loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS PharmSciTech. 2019;20(7):297.
Lattuada R, Martini A, Muggetti L. SMEDDS® (self-microemulsifying drug delivery systems) for oral administration of an immunosuppressive drug. Eur J Pharm Sci. 1998;6:S67.
Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9(1):87–93.
Shah N, Carvajal M, Patel C, Infeld M, Malick A. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106(1):15–23.
Rahman MA, Hussain A, Hussain MS, Mirza MA, Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm. 2013;39(1):1–19.
Silva LAD, Almeida SL, Alonso EC, Rocha PB, Martins FT, Freitas LA, Stephania FT, Marcilio SSC, Ricardo NM. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. Int J Pharm. 2018;541(1–2):1–10.
Yeom DW, Son HY, Kim JH, Kim SR, Lee SG, Song SH, Chae BR, Choi YW. Development of a solidified self-microemulsifying drug delivery system (S-SMEDDS) for atorvastatin calcium with improved dissolution and bioavailability. Int J Pharm. 2016;506(1–2):302–11.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Youshia J, Kamel AO, El Shamy A, Mansour S. Design of cationic nanostructured heterolipid matrices for ocular delivery of methazolamide. Int J Nanomed. 2012;7:2483.
Agarry S, Solomon B, Audu T. Optimization of process variables for the batch degradation of phenol by Pseudomonas fluorescence using response surface methodology. Int J Chem Technol. 2010;2(2):33–45.
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–77.
Aboelwafa AA, Makhlouf AI. In vivo evaluation and application of central composite design in the optimization of amisulpride self-emulsifying drug delivery system. Am J Drug Discov Dev. 2012;2(1):1–16.
Madhav KV, Kishan V. Self microemulsifying particles of loratadine for improved oral bioavailability: preparation, characterization and in vivo evaluation. J Pharm Investig. 2017;48(4):497–508.
Bhavsar DS, Patel BN, Patel CN. RP-HPLC method for simultaneous estimation of tenofovir disoproxil fumarate, lamivudine, and efavirenz in combined tablet dosage form. Pharm Methods. 2012;3(2):73–8.
Venkatesh M, Mallesh K. Self-nano emulsifying drug delivery system (SNEDDS) for oral delivery of atorvastatin-formulation and bioavailability studies. J Drug Deliv Ther. 2013;3(3):131–40.
Wei L, Sun P, Nie S, Pan W. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm. 2005;31(8):785–94.
Gonzalez-Mira E, Egea M, Souto E, Calpena A, Garcia M. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2011;22(4): 045101. https://doi.org/10.1088/0957-4484/22/4/045101.
Mateen QS, Khan SU, Islam DT, Khan NA, Farooqi IH. Copper (II) removal in a column reactor using electrocoagulation: parametric optimization by RSM using central composite design. Water Environ Res. 2020;92(9):1350–62.
Sharifpour E, Ghaedi M, Asfaram A, Farsadrooh M, Dil EA, Javadian H. Modeling and optimization of ultrasound-assisted high performance adsorption of Basic Fuchsin by starch-capped zinc selenide nanoparticles/AC as a novel composite using response surface methodology. Int J Biol Macromol. 2020;152:913–21.
Montazam SH, Salatin S, Alami-Milani M, Naderi A, Jelvehgari M. Expert design and desirability function approach for the development of diazepam thermally sensitive rectal gel. Ther Deliv. 2020;11(1):813–30.
Ahsan MN, Verma PRP. Development, optimization and pharmacodynamic assessment of olanzapine based lipidic SNEDDS for proficient management of psychosis. J Pharm Investig. 2017;47(5):395–411.
Das SS, Singh A, Kar S, Ghosh R, Pal M, Fatima M, Neeru S, Sandeep KS. Application of QbD framework for development of self-emulsifying drug delivery systems. Pharmaceutical Quality by Design. Elsevier. 2019; 297–350.
Marasini N, Yan YD, Poudel BK, Choi HG, Yong CS, Kim JO. Development and optimization of self-nanoemulsifying drug delivery system with enhanced bioavailability by Box-Behnken design and desirability function. J Pharm Sci. 2012;101(12):4584–96.
Abou-Taleb NH, El-Enany NM, El-Sherbiny DT, El-Subbagh HI. Digitally enhanced thin layer chromatography for simultaneous determination of norfloxacin and tinidazole with the aid of Taguchi orthogonal array and desirability function approach: greenness assessment by analytical eco-scale. J Sep Sci. 2019;43(6):1195–202.
Yan L, Zhang S, Liu H, Wang W, Chen P, Li H. Optimization of magnetosome production by Acidithiobacillus ferrooxidans using desirability function approach. Mater Sci Eng C Mater Biol Appl. 2016;59:731–9.
Liu Y, Zhang P, Feng N, Zhang X, Wu S, Zhao J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2009;365(1–2):136–42.
Visetvichaporn V, Kim KH, Jung K, Cho YS, Kim DD. Formulation of self-microemulsifying drug delivery system (SMEDDS) by D-optimal mixture design to enhance the oral bioavailability of a new cathepsin K inhibitor (HL235). Int J Pharm. 2020;573: 118772. https://doi.org/10.1016/j.ijpharm.2019.118772.
Khan I, Gothwal A, Sharma AK, Qayum A, Singh SK, Gupta U. Biodegradable nano-architectural PEGylated approach for the improved stability and anticancer efficacy of bendamustine. Int J Biol Macromol. 2016;92:1242–51.
Valicherla GR, Dave KM, Syed AA, Riyazuddin M, Anand PG, Akhilesh S, Wahajuddin, Kalyan M, Deepak D, Jiaur RG. Formulation optimization of docetaxel loaded self-emulsifying drug delivery system to enhance bioavailability and anti-tumor activity. Sci Rep. 2016;6:26895. https://doi.org/10.1038/srep26895.
Li F, Song S, Guo Y, Zhao Q, Zhang X, Pan W, Yang X. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug lornoxicam. Drug Deliv. 2015;22(4):487–98.
Narender D, Kishan V. Improved anti-hyper lipidemic activity of rosuvastatin calcium via lipid nanoparticles: pharmacokinetic and pharmacodynamic evaluation. Eur J Pharm Biopharm. 2017;110:45–57.
Krstić M, Medarević Đ, Đuriš J, Svetlana I. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid nanocarriers for drug targeting. pp. 473–508. 2018. William Andrew Publishing. https://doi.org/10.1016/B978-0-12-813687-4.00012-8.
Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov Today. 2008;13(13–14):606–12.
Vraníková B, Gajdziok J, Vetchý D. Determination of flowable liquid retention potential of aluminometasilicate carrier for liquisolid systems preparation. Pharm Dev Technol. 2015;20(7):839–44.
Narala A, Guda S, Veerabrahma K. Lipid nanoemulsions of rebamipide: formulation, characterization, and in vivo evaluation of pharmacokinetic and pharmacodynamic effects. AAPS PharmSciTech. 2019;20(1):26.
Kim DS, Cho JH, Park JH, Kim JS, Song ES, Kwon J, Jin SG, Kim KS, Choi HG, Kim DW. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int J Nanomed. 2019;14:4949–60.
Tung NT, Tran CS, Nguyen HA, Nguyen TL, Chi SC, Nguyen DD. Development of solidified self-microemulsifying drug delivery systems containing l-tetrahydropalmatine: design of experiment approach and bioavailability comparison. Int J Pharm. 2018;537(1–2):9–21.
Hosny KM, Alhakamy NA, Almodhwahi MA, Mallesk K, Alshaimaa MA, Samar SE. Self-nanoemulsifying system loaded with sildenafil citrate and incorporated within oral lyophilized flash tablets: preparation, optimization, and in vivo evaluation. Pharmaceutics. 2020;12(11):1124.
Yin K, Meng X, Dong P, Ding T, Shen L, Zhang L, Renfang Z, Wemin C, Hongzhou L. A simple, rapid, economical, and practical method for the determination of efavirenz in plasma of Chinese AIDS patients by reverse phase high-performance liquid chromatography with ultraviolet detector. Biosci Trends. 2014;8(4):227–34.
Irini M, Panagiotis B, Andrea S, Ioannis N. The influence of surfactant HLB and oil/surfactant ratio on the formation and properties of self-emulsifying pellets and microemulsion reconstitution. AAPS PharmSciTech. 2012;13(4):1319–30.
Kamboj S, Rana V. Quality-by-design based development of a self-microemulsifying drug delivery system to reduce the effect of food on nelfinavir mesylate. Int J Pharm. 2016;501(1–2):311–25.
Sriwidodo S, Subroto T, Maksum IP, Wathoni N, Rostinawati T, Ulya H, Indah UP. Optimization of secreted recombinant human epidermal growth factor production using pectate lyase B from Escherichia coli BL21 (DE3) by central composite design and its production in high cell density culture. J Pharm Bioall Sci. 2019;11(8):S562–6.
Dudhipala N, Veerabrahma K. Pharmacokinetic and pharmacodynamic studies of nisoldipine-loaded solid lipid nanoparticles developed by central composite design. Drug Dev Ind Pharm. 2015;41(12):1968–77.
Varshosaz J, Ghaffari S, Khoshayand MR, Atyabi F, Azarmi S, Kobarfard F. Development and optimization of solid lipid nanoparticles of amikacin by central composite design. J Liposome Res. 2010;20(2):97–104.
Madhav KV, Kishan V. Improvement of anti-hyperlipidemic activity and oral bioavailability of fluvastatin via solid self-microemulsifying systems and comparative with liquisolid formulation. Curr Drug Deliv. 2018;15(9):1245–60.
Rangaraj N, Shah S, Maruti AJ, Sravanthi RP, Hanumanth SC, Sujatha D, Sunitha S. Quality by design approach for the development of self-emulsifying systems for oral delivery of febuxostat: pharmacokinetic and pharmacodynamic evaluation. AAPS PharmSciTech. 2019;20:267.
Katla VM, Veerabrahma K. Cationic solid self micro emulsifying drug delivery system (SSMED) of losartan: formulation development, characterization and in vivo evaluation. J Drug Deliv Sci Tec. 2016;35:190–9.
Acknowledgements
The authors are indebted to M/s Aizant Drug Research Pvt. Ltd., India for providing drug sample of efavirenz. The authors are also thankful to M/s Abitec Corporation, USA, and M/s Gattefosse, France for supplying free samples of vehicles. The authors are thankful to M/s Fuji Chemicals, Japan, M/s Grace & Co for contributing free samples of solid carriers. And also to M/s ACG associated capsules Pvt. Ltd., India to supply free empty hard gelatin capsules.
Author information
Authors and Affiliations
Contributions
The research idea was proposed by Prof. V. Kishan (corresponding author). The experimental work was carried out by Mr. Thota Sunil Kumar, a master student. Manuscript was drafted by Mr. Thota Sunil Kumar. The animal studies and software applications were supported by Narendar Dudhipala and Venumadhav Katla. Prof. V. Kishan interpreted the data and supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thota, S.K., Dudhipala, N., Katla, V. et al. Cationic Solid SMEDDS of Efavirenz for Improved Oral Delivery: Development by Central Composite Design, In Vitro and In Vivo Evaluation. AAPS PharmSciTech 24, 38 (2023). https://doi.org/10.1208/s12249-022-02495-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02495-3