Abstract
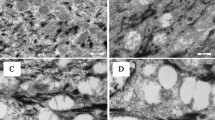
UV radiation can cause damages, such as erythema, skin photoaging, and carcinogenesis. The adoption of protective measures against sun exposure is essential to prevent these damages, and the interest in using natural substances as an alternative for photoprotection is growing. Thus, hesperetin with antioxidant, anti-inflammatory, and anticancer properties is a promising substance to be used with photochemopreventive action and to protect the skin from damage induced by UV radiation. Therefore, the present study aimed to develop a topical formulation based on AAMVPC gel containing hesperetin and evaluate its photoprotective effect on the skin of rats exposed to UVA-UVB radiation. The animals were submitted to the irradiation protocol UVA-UVB, and at the end, erythema, lipid peroxidation, and activity of the antioxidant enzyme catalase and superoxide dismutase were evaluated. Additionally, it evaluated the activity of myeloperoxidase and histological changes. The formulation presented a rheological and spreadability profile suitable for cutaneous application. In vivo results demonstrated that the topical formulation of AAMVPC gel containing hesperetin at a concentration of 10% protected the skin from damage induced by UVA-UVB radiation, with the absence of erythema, lipid lipoperoxidation, and inflammation (low myeloperoxidase activity), and increased catalase and superoxide dismutase activities. The morphology and architecture of the dermo-epidermal tissue of these animals were like those observed under normal conditions (non-irradiated animals). Thus, the results showed that hesperetin was able to protect the animals’ skin against UV radiation–induced skin damage and the protection mechanisms may be related to the antioxidant and anti-inflammatory properties of this natural product.
Graphical Abstract











Similar content being viewed by others
References
Baccarin T, Mitjans M, Ramos D, Lemos-Senna E, Vinardell MP. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J Photochem Photobiol B Biol [Internet]. Elsevier B.V.; 2015;153:127–36. Available from: https://doi.org/10.1016/j.jphotobiol.2015.09.005
Türker H. The effect of ultraviolet radiation of pancreatic exocrine cells in mole rats: An ultrastructural study. J Radiat Res Appl Sci. 2015;8:49–54.
Batista CM, Alves AVF, Queiroz LA, Lima BS, Filho RNP, Araújo AAS, et al. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J Photochem Photobiol B Biol [Internet]. Elsevier B.V; 2018;180:198–207. Available from: https://doi.org/10.1016/j.jphotobiol.2018.01.028
Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev [Internet]. Elsevier; 2020;59:101036. Available from: https://doi.org/10.1016/j.arr.2020.101036
Serafini MR, Detoni CB, Menezes PDP, Pereira Filho RN, Fortes VS, Vieira MJF, et al. UVA-UVB Photoprotective Activity of Topical Formulations Containing Morinda citrifolia Extract. Biomed Res Int. Hindawi Publishing Corporation; 2014;2014.
Petruk G, Giudice R Del, Rigano MM, Monti DM. Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev. 2018;2018.
Sajo MEJ, Kim CS, Kim SK, Shim KY, Kang TY, Lee KJ. Antioxidant and Anti-Inflammatory Effects of Shungite against Ultraviolet B Irradiation-Induced Skin Damage in Hairless Mice. Oxid Med Cell Longev. 2017;2017.
Nobile V, Michelotti A, Cestone E, Caturla N, Castillo J, Benavente-García O, et al. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr Res. 2016;60.
Liu X, Zhang R, Shi H, Li X, Li Y, Taha A, et al. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol Med Rep. 2018;17:7227–37.
Zhou Y, Yang W, Li Z, Luo D, Li W, Zhang Y, et al. Moringa oleifera stem extract protect skin keratinocytes against oxidative stress injury by enhancement of antioxidant defense systems and activation of PPARα. Biomed Pharmacother [Internet]. Elsevier; 2018;107:44–53. Available from: https://doi.org/10.1016/j.biopha.2018.07.152
Cho BO, Che DN, Shin JY, Kang HJ, Kim JH, Kim HY, et al. Ameliorative effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice. Biomed Pharmacother [Internet]. Elsevier Masson SAS; 2017;95:264–74. Available from: https://doi.org/10.1016/j.biopha.2017.07.159
Nery ÉM, Martinez RM, Velasco MVR, Baby AR. A short review of alternative ingredients and technologies of inorganic UV filters. J Cosmet Dermatol [Internet]. John Wiley & Sons, Ltd; 2021 [cited 2022 May 15];20:1061–5. Available from: https://doi.org/10.1111/jocd.13694
Schneider SL, Lim HW. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol Photoimmunol Photomed [Internet]. Photodermatol Photoimmunol Photomed; 2019 [cited 2022 May 15];35:442–6. Available from: https://pubmed.ncbi.nlm.nih.gov/30444533/
Schneider SL, Lim HW. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol [Internet]. J Am Acad Dermatol; 2019 [cited 2021 Dec 7];80:266–71. Available from: https://pubmed.ncbi.nlm.nih.gov/29981751/
Narla S, Lim HW. Sunscreen: FDA regulation, and environmental and health impact. Photochem Photobiol Sci [Internet]. Royal Society of Chemistry; 2020 [cited 2021 Dec 7];19:66–70. Available from: https://www.researchgate.net/publication/337461311_Sunscreen_FDA_Regulation_and_Environmental_and_Health_Impact
Ferreira de Oliveira JMP, Santos C, Fernandes E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine [Internet]. Elsevier; 2020;73:152887. Available from: https://doi.org/10.1016/j.phymed.2019.152887
Ding HW, Huang AL, Zhang YL, Li B, Huang C, Ma T tao, et al. Design, synthesis and biological evaluation of hesperetin derivatives as potent anti-inflammatory agent. Fitoterapia. 2017;121:212–22.
Samie A, Sedaghat R, Baluchnejadmojarad T, Roghani M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci [Internet]. Elsevier Inc; 2018;210:132–9. Available from: https://doi.org/10.1016/j.lfs.2018.08.074
Aswar M, Kute P, Mahajan S, Mahajan U, Nerurkar G, Aswar U. Protective effect of hesperetin in rat model of partial sciatic nerve ligation induced painful neuropathic pain: An evidence of anti-inflammatory and anti-oxidative activity. Pharmacol Biochem Behav [Internet]. Elsevier B.V.; 2014;124:101–7. Available from: https://doi.org/10.1016/j.pbb.2014.05.013
Bodduluru LN, Kasala ER, Barua CC, Karnam KC, Dahiya V, Ellutla M. Antiproliferative and antioxidant potential of hesperetin against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Chem Biol Interact [Internet]. Elsevier Ltd; 2015;242:345–52. Available from: https://doi.org/10.1016/j.cbi.2015.10.020
Tan YQ, Chiu-Leung LC, Lin S mei, Leung LK. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp Biochem Physiol Part - C Toxicol Pharmacol [Internet]. Elsevier; 2018;210:57–64. Available from: https://doi.org/10.1016/j.cbpc.2018.05.007
Wang Y, Liu S, Dong W, Qu X, Huang C, Yan T, et al. Combination of hesperetin and platinum enhances anticancer effect on lung adenocarcinoma. Biomed Pharmacother [Internet]. Elsevier; 2019;113:108779. Available from: https://doi.org/10.1016/j.biopha.2019.108779
Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother [Internet]. Elsevier Masson SAS; 2017;88:124–33. Available from: https://doi.org/10.1016/j.biopha.2016.11.089
Khan A, Ikram M, Hahm JR, Kim MO. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants. 2020;9:1–15.
Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci [Internet]. Elsevier B.V.; 2015;137:125–32. Available from: https://doi.org/10.1016/j.lfs.2015.07.014
Petrova A, Davids LM, Rautenbach F, Marnewick JL. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J Photochem Photobiol B Biol [Internet]. Elsevier B.V.; 2011;103:126–39. Available from: https://doi.org/10.1016/j.jphotobiol.2011.02.020
Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74.
Daneluz J, da Silva Favero J, dos Santos V, Weiss-Angeli V, Gomes LB, Mexias AS, et al. The influence of different concentrations of a natural clay material as active principle in cosmetic formulations. Mater Res. 2020;23.
Zanini M. Gel de ácido tricloroacético - Uma nova técnica para um antigo ácido. Med Cutan Ibero Lat Am. 2007;35:14–7.
Carvalho YMBG, Menezes PP, Sousa BMH, Lima BS, Trindade IAS, Serafini MR, et al. Inclusion complex between β-cyclodextrin and hecogenin acetate produces superior analgesic effect in animal models for orofacial pain. Biomed Pharmacother. 2017;93:754–62.
Francisconi RS, Maquera-Huacho PM, Tonon CC, Calixto GMF, de Cássia Orlandi Sardi J, Chorilli M, et al. Terpinen-4-ol and nystatin co-loaded precursor of liquid crystalline system for topical treatment of oral candidiasis. Sci Rep [Internet]. Nature Publishing Group UK; 2020;10:1–12. Available from: https://doi.org/10.1038/s41598-020-70085-z
Menezes P, Frank LA, Lima B, Carvalho Y, Serafini M, Quintans-Júnior L, et al. Hesperetin-loaded lipid-core nanocapsules in polyamide: a new textile formulation for topical drug delivery. Int J Nanomedicine [Internet]. 2017 [cited 2017 Sep 13];Volume 12:2069–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28352176
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem Academic Press. 1976;72:248–54.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Aebi H. [13] Catalase in Vitro. Methods Enzymol. 1984;105:121–6.
Draper HH, Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990;186:421–31.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys Academic Press. 1959;82:70–7.
Marchiori MCL, Rigon C, Camponogara C, Oliveira SM, Cruz L. Hydrogel containing silibinin-loaded pomegranate oil based nanocapsules exhibits anti-inflammatory effects on skin damage UVB radiation-induced in mice. J Photochem Photobiol B Biol. Elsevier B.V.; 2017;170:25–32.
Dimaki A, Kyriazi M, Leonis G, Sfiniadakis I, Papaioannou GT, Ioannou E, et al. Diabetic skin and UV light: Protection by antioxidants. Eur J Pharm Sci. Elsevier B.V.; 2019;127:1–8.
Guo J, Tang W, Lu S, Fang Z, Tu K, Zheng M. Solubility improvement of hesperetin by using different octenyl succinic anhydride modified starches. Lwt [Internet]. Elsevier Ltd; 2018;95:255–61. Available from: https://doi.org/10.1016/j.lwt.2018.04.056
Ficarra R, Tommasini S, Raneri D, Calabrò ML, Di Bella MR, Rustichelli C, et al. Study of flavonoids/β-cyclodextrins inclusion complexes by NMR, FT-IR, DSC. X-ray investigation J Pharm Biomed Anal. 2002;29:1005–14.
Lima B dos S, Campos C de A, da Silva Santos ACR, Santos VCN, Trindade G das GG, Shanmugam S, et al. Development of morin/hydroxypropyl-β-cyclodextrin inclusion complex: Enhancement of bioavailability, antihyperalgesic and anti-inflammatory effects. Food Chem Toxicol [Internet]. Elsevier; 2019;126:15–24. Available from: https://doi.org/10.1016/j.fct.2019.01.038
Frank LA, Sandri G, D’Autilia F, Contri R V, Bonferoni MC, Caramella C, et al. Chitosan gel containing polymeric nanocapsules: a new formulation for vaginal drug delivery. Int J Nanomedicine [Internet]. Dove Press; 2014 [cited 2017 Sep 15];9:3151–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25061292
Frank LA, Chaves PS, D’Amore CM, Contri R V., Frank AG, Beck RCR, et al. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur J Pharm Biopharm [Internet]. 2017 [cited 2017 Oct 29];114:202–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28161547
Pegoraro NS, Barbieri A V., Camponogara C, Mattiazzi J, Brum ES, Marchiori MCL, et al. Nanoencapsulation of coenzyme Q10 and vitamin E acetate protects against UVB radiation-induced skin injury in mice. Colloids Surfaces B Biointerfaces [Internet]. 2017 [cited 2017 Nov 10];150:32–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27870992
Hewitt J, Dahms GH. Rheology : its effect on physical SPFs. SPC Soap, Perfum Cosmet. 1996;69.
Gaspar LR, Maia Campos PMBG. Rheological behavior and the SPF of sunscreens. Int J Pharm [Internet]. Int J Pharm; 2003 [cited 2022 May 15];250:35–44. Available from: https://pubmed.ncbi.nlm.nih.gov/12480271/
Felippim EC, Marcato PD, Maia Campos PMBG. Development of Photoprotective Formulations Containing Nanostructured Lipid Carriers: Sun Protection Factor, Physical-Mechanical and Sensorial Properties. AAPS PharmSciTech [Internet]. AAPS PharmSciTech; 2020 [cited 2022 May 15];21. Available from: https://pubmed.ncbi.nlm.nih.gov/33161472/
Alencar Filho JMT de, Sampaio PA, Carvalho IS de, Guimarães AL, Amariz IA e, Pereira ECV, et al. Flavonoid enriched extract of Alternanthera brasiliana with photoprotective effect: Formulation development and evaluation of quality. Ind Crops Prod. Elsevier BV; 2020;149:112371.
Mayba JN, Gooderham MJ. A guide to topical vehicle formulations. J Cutan Med Surg. SAGE Publications Inc.; 2018;22:207–12.
Kelmann RG, Colombo M, Nunes RJ, Simões CMO, Koester LS. Nanoemulsion-Loaded Hydrogels for Topical Administration of Pentyl Gallate. AAPS PharmSciTech. 2018;19:2672–8.
Das S, Das J, Paul A, Samadder A, Khuda-Bukhsh AR. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet b-induced reactive oxygen species and DNA damage. JAMS J Acupunct Meridian Stud [Internet]. Elsevier Korea LLC; 2013;6:252–62. Available from: https://doi.org/10.1016/j.jams.2013.07.002
Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol. 2013;45:395–8.
Divya SP, Wang X, Pratheeshkumar P, Son YO, Roy RV, Kim D, et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol Appl Pharmacol [Internet]. Elsevier B.V.; 2015;284:92–9. Available from: https://doi.org/10.1016/j.taap.2015.02.003
Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer [Internet]. Nat Rev Cancer; 2004 [cited 2021 Nov 8];4:23–35. Available from: https://pubmed.ncbi.nlm.nih.gov/14681688/
Dröge W. Free radicals in the physiological control of cell function. Physiol Rev [Internet]. Physiol Rev; 2002 [cited 2021 Nov 8];82:47–95. Available from: https://pubmed.ncbi.nlm.nih.gov/11773609/
F’guyer S, Afaq F, Mukhtar H. Photochemoprevention of skin cancer by botanical agents. Photodermatol Photoimmunol Photomed [Internet]. Photodermatol Photoimmunol Photomed; 2003 [cited 2021 Nov 8];19:56–72. Available from: https://pubmed.ncbi.nlm.nih.gov/12945805/
Bedirli N, Bagriacik EU, Yilmaz G, Ozkose Z, Kavutçu M, Cavunt Bayraktar A, et al. Sevoflurane exerts brain-protective effects against sepsis-associated encephalopathy and memory impairment through caspase 3/9 and Bax/Bcl signaling pathway in a rat model of sepsis. J Int Med Res. SAGE Publications Ltd; 2018;46:2828–42.
Pratheeshkumar P, Kuttan G. Vernolide-A, a sesquiterpene lactone from Vernonia cinerea, induces apoptosis in B16F–10 melanoma cells by modulating p53 and caspase-3 gene expressions and regulating NF-κB-mediated bcl-2 activation. Drug Chem Toxicol. 2011;34:261–70.
SD S, SM M, SK K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther [Internet]. Mol Cancer Ther; 2007 [cited 2021 Nov 2];6:995–1005. Available from: https://pubmed.ncbi.nlm.nih.gov/17363493/
S S, M R, A P, B A, R M, SR C, et al. Molecular mechanism underlying hesperetin-induced apoptosis by in silico analysis and in prostate cancer PC-3 cells. Asian Pac J Cancer Prev [Internet]. Asian Pac J Cancer Prev; 2013 [cited 2021 Nov 2];14:4347–52. Available from: https://pubmed.ncbi.nlm.nih.gov/23992001/
Acknowledgements
The authors thank CLQM (Center of Multi-users Chemistry Laboratories) from Federal University of Sergipe for the analysis support. The authors also thank the FAPITEC/SE and CNPq (Brazil) for their financial support.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Finance Code 001].
Author information
Authors and Affiliations
Contributions
The authors’ role was based on the following criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work—Tatianny de Araújo Andrade, Luana Heimfarth, Danillo Menezes dos Santos, Márcio Roberto Viana dos Santos, Ricardo Luiz Cavalcanti de Albuquerque-Júnior, Agenor Gomes dos Santos-Neto, Guilherme Rodolfo Souza de Araujo, Ana Amélia Moreira Lira, Saulo Santos Matos, Luiza Abrahão Frank, Thallita Kelly Rabelo, Lucindo José Quintans-Júnior, Jullyana de Souza Siqueira Quintans, Adriano Antunes de Souza Araujo, and Mairim Russo Serafini.
- Drafting the work or revising it critically for important intellectual content—Tatianny de Araújo Andrade, Luana Heimfarth, Márcio Roberto Viana dos Santos, Ricardo Luiz Cavalcanti de Albuquerque-Júnior, Agenor Gomes dos Santos-Neto, Luiza Abrahão Frank, and Mairim Russo Serafini.
- Final approval of the version to be published—Tatianny de Araújo Andrade, Luana Heimfarth, Danillo Menezes dos Santos, Márcio Roberto Viana dos Santos, Ricardo Luiz Cavalcanti de Albuquerque-Júnior, Agenor Gomes dos Santos-Neto, Guilherme Rodolfo Souza de Araujo, Ana Amélia Moreira Lira, Saulo Santos Matos, Luiza Abrahão Frank, Thallita Kelly Rabelo, Lucindo José Quintans-Júnior, Jullyana de Souza Siqueira Quintans, Adriano Antunes de Souza Araujo, and Mairim Russo Serafini.
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved—Tatianny de Araújo Andrade and Mairim Russo Serafini.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Araújo Andrade, T., Heimfarth, L., dos Santos, D.M. et al. Hesperetin-Based Hydrogels Protect the Skin against UV Radiation-Induced Damage. AAPS PharmSciTech 23, 170 (2022). https://doi.org/10.1208/s12249-022-02323-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02323-8