Abstract
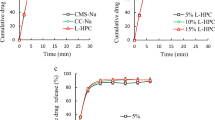
Omeprazole is a widely used over-the-counter (20 mg) proton pump inhibitor, usually supplied as oral enteric-coated pellets intended to release at pH 5.5 and higher; however, it is sensitive to acidic pH. The likelihood of elevated gastric pH in practice is very high for patients; thus, the aim of this study was to investigate the effect of elevated pH on the performance of commercial omeprazole pellets. Commercial enteric-coated delayed-release pellets were tested with water uptake-weight loss (WU-WL) test at pH range between 1.2 and 4.5 in addition to “gastric” (pH 1.2 or 4.5) and “intestinal” (pH 7.4) phase dissolution tests. The range of physical characteristics of pellets was determined with a single pellet size and sedimentation time measurement, followed by the application of modified Stokes’ Law equation. The coefficient of variation of pellet size and density, and volume-density determination coefficient (R2) as descriptors of coating thickness and microstructure variability, degree of ionisation of enteric polymers, aqueous solubility and molecular weight of plasticisers have been found useful to explain commercial delayed-release pellets behaviour during WU-WL and dissolution test. Investigated commercial delayed-release pellets demonstrated pH-dependent WU-WL results. “Gastric phase” dissolution testing of pellets at pH 4.5 showed the highest omeprazole degradation (48.1%) for Nosch Labs, intermediate values of dose loss (23.4% and 17.1%) for Teva and UQUIFA delayed-release pellets, respectively. Lab Liconsa pellets have been found as the least susceptible (3.2% of dose loss). Additionally, “gastric phase” dissolution test at pH 4.5 significantly influenced omeprazole release during the “intestinal phase”. The risk of inadequate therapy associated with intake of investigated enteric-coated delayed-release pellets at elevated gastric pH has been found as minimal for Lab Liconsa and has increased from UQUIFA and Teva to Nosh Labs pellets.








Similar content being viewed by others
References
Lovgren KI, Pilbrant AG, Yasumura M, Morigaki S, Oda M, Ohishi N. Pharmaceutical preparation for oral use (patent No US4786505). AstraZeneca; 1986.
Sachs G, Scott D, Reuben M. Omeprazole and the gastric mucosa. Digestion. 1990;47(Suppl. 1):35–8.
Solana MJ, Lopez-Herce J. Pharmacokinetics of intravenous omeprazole in critically ill paediatric patients. Eur J Clin Pharmacol. 2010;66(4):323–30. https://doi.org/10.1007/s00228-009-0774-9.
Bengtsson IS, Lovgren KI. Pharmaceutical formulation of omeprazole (patent No US5690960). AstraZeneca; 1993.
Bergstrand P, Lovgren KI. Multiple unit pharmaceutical preparation (patent No US5753265). AstraZeneca; 1994.
Bergstrand P, Lovgren KI. Multiple unit tableted dosage form of omeprazole (patent No US5817338). AstraZeneca; 1994.
Kallstrom LA, Nygren MA. Omeprazole magnesium salt form (patent No US5900424). AstraZeneca; 1993.
Lovqvist K, Sunden G, Noreland D, Ymen I. Crystalline form of omeprazole (patent No US6150380). AstraZeneca; 1998.
Anousis N, McManus JW, Banks BN, Zhou L, Liu H. Omeprazole process and compositions thereof (patent No US6166213). Merck Sharp and Dohme Corp; 1998.
McManus JW, Anousis N, Banks BN, Liu H, Zhou L. Omerazole process and compositions thereof (patent No US6191148). Merck Sharp and Dohme Corp; 1998.
Bergstrand P, Wang P. Pharmaceutical formulation comprising omeprazole (patent No US6428810). AstraZeneca; 1998.
Anousis N, McManus JW, Banks BN, Zhou L. Omeprazole process and compositions thereof (patent No US6147103). Merck Sharp and Dohme Corp; 1999.
Erickson M, Gustavsson A, Josefsson L. Chemical process and pharmaceutical formulation (patent No US6403616). AstraZeneca; 1999.
Suedee R, Jantarat C, Lindner W, Viernstein H, Songkro S, Srichana T. Development of a pH-responsive drug delivery system for enantioselective-controlled delivery of racemic drugs. J Control Release. 2010;142(1):122–31. https://doi.org/10.1016/j.jconrel.2009.10.011.
Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28(8):966–72.
Yang R. Physicochemical Studies on Omeprazole: University of Florida; 2002.
Yang R, Schulman SG, Zavala PJ. Acid–base chemistry of omeprazole in aqueous solutions. Anal Chim Acta. 2003;481(1):155–64. https://doi.org/10.1016/s0003-2670(03)00076-x.
El-Badry M, Taha EI, Alanazi FK, Alsarra IA. Study of omeprazole stability in aqueous solution: influence of cyclodextrins. J Drug Del Sci Tech. 2009;19:347–51.
Mathew M, Gupta VD, Bailey RE. Stability of Omeprazole Solutions at Various ph Values as Determined by High-Performance Liquid Chromatography. Drug Dev Ind Pharm. 2008;21(8):965–71. https://doi.org/10.3109/03639049509026660.
Farinha A, Bica A, Martins JM, Pais JP. Dissolution of omeprazole from delayed-release solid oral dosage forms. Drug Dev Ind Pharm. 2000;26(7):785–90. https://doi.org/10.1081/ddc-100101300.
Vervaet C, Baert L, Remon JP. Extrusion-Spheronisation - a Literature-Review. Int J Pharm. 1995;116(2):131–46. https://doi.org/10.1016/0378-5173(94)00311-R.
Chen C-M, Chou JC, Weng T. Omeprazole formulation (patent No US6077541). 1999.
Chen C-M, Chou JC, Weng T. Omeprazole formulation (patent No US6096340). 2000.
Riedel A, Leopold CS. Degradation of omeprazole induced by enteric polymer solutions and aqueous dispersions: HPLC investigations. Drug Dev Ind Pharm. 2005;31(2):151–60. https://doi.org/10.1081/ddc-200047787.
Riedel A, Leopold CS. Quantification of omeprazole degradation by enteric coating polymers: an UV-VIS spectroscopy study. Pharmazie. 2005;60(2):126–30.
Chen C-M, Chou J, Kositprapa U. Omeprazole formulation (patent No US6855366B2). 2005.
Mohylyuk V, Styliari ID, Novykov D, Pikett R, Dattani R. Assessment of the effect of Cellets’ particle size on the flow in a Wurster fluid-bed coater via powder rheology. J Drug Deliv Sci Tec. 2019;54:101320. https://doi.org/10.1016/j.jddst.2019.101320.
Ronchi F, Sereno A, Paide M, Sacré P, Guillaume G, Stéphenne V, et al. Development and evaluation of an omeprazole-based delayed-release liquid oral dosage form. Int J Pharm. 2019;567:118416.
Al-Gousous J, Amidon GL, Langguth P. Toward Biopredictive Dissolution for Enteric Coated Dosage Forms. Mol Pharm. 2016;13(6):1927–36. https://doi.org/10.1021/acs.molpharmaceut.6b00077.
Storpirtis S, Rodrigues D. In vitro evaluation of dissolution properties and degradation products of omeprazole in enteric-coated pellets. Drug Dev Ind Pharm. 1998;24(11):1101–7. https://doi.org/10.3109/03639049809089956.
Moore T, Smith A, Ye W, Toler DY, Westenberger BJ, Lionberger R, et al. Generic omeprazole delayed-release capsules: in vitro performance evaluations. Drug Dev Ind Pharm. 2009;35(8):917–21. https://doi.org/10.1080/03639040802698802.
Agyilirah GA, Banker GS. Polymers for enteric coating applications. Polymers for controlled drug delivery. 3: CRC Press; 1991. p. 39-66.
Shimatani T, Inoue M, Kuroiwa T, Xu J, Tazuma S, Horikawa Y, et al. Acid-suppressive efficacy of a reduced dosage of rabeprazole: comparison of 10 mg twice daily rabeprazole with 20 mg twice daily rabeprazole, 30 mg twice daily lansoprazole, and 20 mg twice daily omeprazole by 24-hr intragastric pH-metry. Dig Dis Sci. 2005;50(7):1202–6. https://doi.org/10.1007/s10620-005-2760-0.
Koziolek M, Schneider F, Grimm M, Modebeta C, Seekamp A, Roustom T, et al. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J Control Release. 2015;220(Pt A):71–8. https://doi.org/10.1016/j.jconrel.2015.10.022.
Phillips JO. Omeprazole solution and method for using same (patent No US5840737). 1996.
Phillips JO. Substituted benzimidazole dosage forms and method of using same (patent No US7399772B2). 2003.
Larsson H, Hakanson R, Mattsson H, Ryberg B, Sundler F, Carlsson E. Omeprazole: its influence on gastric acid secretion, gastrin and ECL cells. Toxicol Pathol. 1988;16(2):267–72. https://doi.org/10.1177/019262338801600220.
Hata S, Arai M, Maruoka D, Tanaka T, Matsumura T, Suzuki T, et al. Intragastric acidity during the first day following administration of low-dose proton pump inhibitors: a randomized crossover study. Clin Res Hepatol Gastroenterol. 2013;37(3):296–301. https://doi.org/10.1016/j.clinre.2012.07.010.
Metz DC, Amer F, Hunt B, Vakily M, Kukulka MJ, Samra N. Lansoprazole regimens that sustain intragastric pH > 6.0: an evaluation of intermittent oral and continuous intravenous infusion dosages. Aliment Pharmacol Ther. 2006;23(7):985–95. https://doi.org/10.1111/j.1365-2036.2006.02850.x.
Patel D, Bertz R, Ren S, Boulton DW, Nagard M. A Systematic Review of Gastric Acid-Reducing Agent-Mediated Drug-Drug Interactions with Orally Administered Medications. Clin Pharmacokinet. 2020;59(4):447–62. https://doi.org/10.1007/s40262-019-00844-3.
Kyivmedpreparat PJSC. Package leaflet (information for the user): Omeprazole 20mg Gastro-resistant Capsules (MA #UA/0966/01/01). 2015.
Chemo Iberica SA. Package leaflet (information for the user): Omeprazole 20mg Gastro-resistant Capsules. 2015.
Public assessment report: Omeprazol 10/20/40 mg gastro-resistant capsules (Chemo Iberica S.A., Barcelona, Spain). Medicines Evaluation Board in the Netherlands; 2009.
Teva Pharmaceutical Industries Ltd. Package leaflet (information for the user): Omeprazole 20mg Gastro-resistant Capsules (MA #UA/15152/01/02). 2017.
Teva UK Ltd. Summary of product characteristics: Omeprazole 10 mg gastro-resistant capsules (MA #PL00289/1469). 2019.
Farmak JSC. Package leaflet (information for the user): Omeprazole 20mg Gastro-resistant Capsules (MA #UA/UA/4310/01/01). 2015.
Mohylyuk V, Patel K, Murnane D, Richardson C, Liu F. Investigation into the internal structure of coated microparticles to support formulation and coating process development. AAPS PharmSci 360; Washington, USA. 2018.
Fu M, Al-Gousous J, Blechar JA, Langguth P. Enteric Hard Capsules for Targeting the Small Intestine: Positive Correlation between In Vitro Disintegration and Dissolution Times. Pharmaceutics. 2020;12(2):123. https://doi.org/10.3390/pharmaceutics12020123.
Liu F, Merchant HA, Kulkarni RP, Alkademi M, Basit AW. Evolution of a physiological pH 6.8 bicarbonate buffer system: application to the dissolution testing of enteric coated products. Eur J Pharm Biopharm. 2011;78(1):151–7. https://doi.org/10.1016/j.ejpb.2011.01.001.
Karkossa F, Klein S. Assessing the influence of media composition and ionic strength on drug release from commercial immediate-release and enteric-coated aspirin tablets. J Pharm Pharmacol. 2017;69(10):1327–40. https://doi.org/10.1111/jphp.12777.
Varum F, Cristina Freire A, Bravo R, Basit AW. OPTICORE, an innovative and accurate colonic targeting technology. Int J Pharm. 2020;119372:119372. https://doi.org/10.1016/j.ijpharm.2020.119372.
Missaghi S, Young C, Fegely K, Rajabi-Siahboomi AR. Delayed release film coating applications on oral solid dosage forms of proton pump inhibitors: case studies. Drug Dev Ind Pharm. 2010;36(2):180–9. https://doi.org/10.3109/03639040903468811.
Khatri P, Desai D, Shelke N, Minko T. Role of plasticizer in membrane coated extended release oral drug delivery system. J Drug Deliv Sci Technol. 2018;44:231–43. https://doi.org/10.1016/j.jddst.2017.12.020.
Bodmeier R, Paeratakul O. Leaching of water-soluble plasticizers from polymeric films prepared from aqueous colloidal polymer dispersions. Drug Dev Ind Pharm. 2008;18(17):1865–82. https://doi.org/10.3109/03639049209046336.
Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends used for the aqueous coating of solid dosage forms: importance of the type of plasticizer. J Control Release. 2004;99(1):1–13. https://doi.org/10.1016/j.jconrel.2004.05.011.
Bando H, McGinity JW. Relationship between drug dissolution and leaching of plasticizer for pellets coated with an aqueous Eudragit S100:L100 dispersion. Int J Pharm. 2006;323(1-2):11–7. https://doi.org/10.1016/j.ijpharm.2006.05.043.
Shibata H, Yoshida H, Izutsu K, Goda Y. Use of bicarbonate buffer systems for dissolution characterization of enteric-coated proton pump inhibitor tablets. J Pharm Pharmacol. 2016;68(4):467–74. https://doi.org/10.1111/jphp.12540.
Scott N, Patel K, Sithole T, Xenofontos K, Mohylyuk V, Liu F. Regulating the pH of bicarbonate solutions without purging gases: Application to dissolution testing of enteric coated tablets, pellets and microparticles. Int J Pharm. 2020;585:119562. https://doi.org/10.1016/j.ijpharm.2020.119562.
Grady H, Elder D, Webster GK, Mao Y, Lin Y, Flanagan T, et al. Industry's View on Using Quality Control, Biorelevant, and Clinically Relevant Dissolution Tests for Pharmaceutical Development, Registration, and Commercialization. J Pharm Sci. 2018;107(1):34–41. https://doi.org/10.1016/j.xphs.2017.10.019.
Acknowledgements
The authors want to thank Maryna Grokholska (Head of Quality Control Department at PrJSC “INDAR”, Kyiv, Ukraine) for her valuable help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 111 kb)
Rights and permissions
About this article
Cite this article
Mohylyuk, V., Yerkhova, A., Katynska, M. et al. Effect of Elevated pH on the Commercial Enteric-Coated Omeprazole Pellets Resistance: Patent Review and Multisource Generics Comparison. AAPS PharmSciTech 22, 188 (2021). https://doi.org/10.1208/s12249-021-02038-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02038-2