Abstract
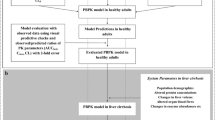
Oseltamivir is a neuraminidase inhibitor widely used to treat and prevent influenza A and B infections, although its safety and pharmacokinetics have not been evaluated in patients with severe hepatic impairment. A physiologically based pharmacokinetic (PBPK) model of the prodrug oseltamivir and its active metabolite, oseltamivir carboxylate (OC), was established and validated to simulate their disposition in adults and predict the exposure in patients with Child-Pugh C cirrhosis (CP-C). The simulated results from PBPK modeling and the observed data after oral administration of various oseltamivir regimens were consistent according to the fold error values of less than 2. Furthermore, the clinical observations published in the literature were comparable with our pharmacokinetic predictions. In patients with CP-C, the oseltamivir Cmax was approximately 2-fold increased, and its AUC was approximately 6-fold higher compared with those in normal subjects. In contrast, the AUC of OC in CP-C patients did not differ significantly from that in normal subjects, whereas its Cmax was reduced by approximately 30% in the patients. Examination of drug exposure in different health conditions indicated that the oseltamivir exposure was significantly increased in conditions with elevated cirrhosis severity, which might be associated with a higher risk of adverse drug effects, e.g., neuropsychiatric adverse events (NPAEs). In conclusion, the pharmacokinetics of oseltamivir and OC were correctly predicted by PBPK modeling. The model further predicted that the pharmacokinetics of oseltamivir might be altered in liver cirrhosis, depending on the degree of severity.




Similar content being viewed by others
References
Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. Jama. 2000;283:1016–24. https://doi.org/10.1001/jama.283.8.1016.
He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999;37:471–84. https://doi.org/10.2165/00003088-199937060-00003.
Hama R. Fatal neuropsychiatric adverse reactions to oseltamivir: case series and overview of causal relationships. Inl J Risk Safe Med. 2008;20:5–36. https://doi.org/10.3233/JRS-2008-0431.
Izumi Y, Tokuda K, O’Dell KA, Zorumski CF, Narahashi T. Neuroexcitatory actions of Tamiflu and its carboxylate metabolite. Neurosci Lett. 2007;426:54–8. https://doi.org/10.1016/j.neulet.2007.08.054.
Morimoto K, Nakakariya M, Shirasaka Y, Kakinuma C, Fujita T, Tamai I, et al. Oseltamivir (Tamiflu) efflux transport at the blood-brain barrier via P-glycoprotein. Drug Metab Dispos. 2008;36:6–9. https://doi.org/10.1124/dmd.107.017699.
Ose A, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Fujita T, et al. P-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrier. Drug Metab Dispos. 2008;36:427–34. https://doi.org/10.1124/dmd.107.018556.
Jhee SS, Yen M, Ereshefsky L, Leibowitz M, Schulte M, Kaeser B, et al. Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers. Antimicrob Agents Chemother. 2008;52:3687–93. https://doi.org/10.1128/AAC.00327-08.
L’Huillier AG, Ing Lorenzini K, Crisinel P-A, Rebsamen MC, Fluss J, Korff CM, et al. ABCB1 polymorphisms and neuropsychiatric adverse events in oseltamivir-treated children during influenza H1N1/09 pandemia. Pharmacogenomics. 2011;12:1493–501. https://doi.org/10.2217/pgs.11.91.
Food and Drug Administration.Tamiflu pharmacology/toxicology NDA/BLA review and evaluation.2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/021246Orig1s045_021087Orig1s062PharmR.pdf. Accessed 2 Oct 2019.
Weiser T, Breidenbach A. Oseltamivir is devoid of specific behavioral and other central nervous system effects in juvenile rats at supratherapeutic oral doses. Int J Virol. 2009. https://doi.org/10.3923/ijv.2009.119.130.
Freichel C, Breidenbach A, Gand L, Toot J, Weiser T, Körner A, et al. Lack of unwanted effects of oseltamivir carboxylate in juvenile rats after subcutaneous administration. Basic Clin Pharmacol Toxicol. 2012;110:551–3. https://doi.org/10.1111/j.1742-7843.2011.00840.x.
Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. https://doi.org/10.1016/S0140-6736(08)60383-9.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206. https://doi.org/10.1016/S0140-6736(08)60383-9.
Shi J, Wang X, Nguyen J, Wu AH, Bleske BE, Zhu H-J. Sacubitril is selectively activated by carboxylesterase 1 (CES1) in the liver and the activation is affected by CES1 genetic variation. Drug Metab Dispos. 2016;44:554–9. https://doi.org/10.1124/dmd.115.068536.
Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147. https://doi.org/10.1007/s00228-008-0553-z.
Oo C, Snell P, Liu B, Martin D, Simkins T, Small I, et al. No dose adjustment of an anti-influenza prodrug oseltamivir is required in patients with hepatic impairment. In: 4th European Congress of Chemotherapy and Infection. Paris: France; 2002.
Snell P, Dave N, Wilson K, Rowell L, Weil A, Galitz L, et al. Lack of effect of moderate hepatic impairment on the pharmacokinetics of oral oseltamivir and its metabolite oseltamivir carboxylate. Br J Clin Pharmacol. 2005;59:598–601. https://doi.org/10.1111/j.1365-2125.2005.02340.x.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. https://doi.org/10.1146/annurev-pharmtox-010510-100540.
Yang Q, Zhai Y, Chen L, Zhang T, Yan Y, Meng T, et al. Whole-body physiology-based pharmacokinetics of caspofungin for general patients, intensive care unit patients and hepatic insufficiency patients. Acta Pharmacol Sin. 2018;39:1533–43. https://doi.org/10.1038/aps.2017.176.
Rasool MF, Khalil F, Läer S. Optimizing the clinical use of carvedilol in liver cirrhosis using a physiologically based pharmacokinetic modeling approach. Eur J Drug Metab Pharmacokinet. 2017;42:383–96. https://doi.org/10.1007/s13318-016-0353-2.
Jogiraju VK, Avvari S, Gollen R, Taft DR. Application of physiologically based pharmacokinetic modeling to predict drug disposition in pregnant populations. Biopharm Drug Dispos. 2017;38:426–38. https://doi.org/10.1002/bdd.2081.
Parrott N, Davies B, Hoffmann G, Koerner A, Lave T, Prinssen E, et al. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin Pharmacokinet. 2011;50:613–23. https://doi.org/10.2165/11592640-000000000-00000.
Oo C, Snell P, Barrett J, Dorr A, Liu B, Wilding I. Pharmacokinetics and delivery of the anti-influenza prodrug oseltamivir to the small intestine and colon using site-specific delivery capsules. Int J Pharm. 2003;257:297–9. https://doi.org/10.1016/s0378-5173(03)00164-9.
Shi D, Yang J, Yang D, LeCluyse EL, Black C, You L, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319:1477–84. https://doi.org/10.1124/jpet.106.111807.
Hsueh C, Hsu V, Zhao P, Zhang L, Giacomini K, Huang S. PBPK modeling of the effect of reduced kidney function on the pharmacokinetics of drugs excreted renally by organic anion transporters. Clin Pharmacol Ther. 2018;103:485–92. https://doi.org/10.1002/cpt.750.
Boberg M, Vrana M, Mehrotra A, Pearce RE, Gaedigk A, Bhatt DK, et al. Age-dependent absolute abundance of hepatic carboxylesterases (CES1 and CES2) by LC-MS/MS proteomics: application to PBPK modeling of oseltamivir in vivo pharmacokinetics in infants. Drug Metab Dispos. 2017;45:216–23. https://doi.org/10.1124/dmd.116.072652.
Massarella JW, He GZ, Dorr A, Nieforth K, Ward P, Brown A. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64–0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol. 2000;40:836–43. https://doi.org/10.1177/00912700022009567.
Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016;6:430–40. https://doi.org/10.1016/j.apsb.2016.04.004.
Lukacova V, Lave T, Fraczkiewicz G, Bolger M, Woltosz W. General approach to calculation of tissue: plasma partition coefficients for physiologically based pharmacokinetic (PBPK) modeling. Toxicol in Vitro. 2008;22:457–67.
Yang J, Shi D, Yang D, Song X, Yan B. Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007;72:686–94. https://doi.org/10.1124/mol.107.036889.
Prasad B, Bhatt DK, Johnson K, Chapa R, Chu X, Salphati L, et al. Abundance of phase 1 and 2 drug-metabolizing enzymes in alcoholic and hepatitis C cirrhotic livers: a quantitative targeted proteomics study. Drug Metab Dispos. 2018;46:943–52. https://doi.org/10.1124/dmd.118.080523.
d’Esposito F, Nebot N, Edwards RJ, Murray M. Impaired irinotecan biotransformation in hepatic microsomal fractions from patients with chronic liver disease. Br J Clin Pharmacol. 2010;70:400–8. https://doi.org/10.1111/j.1365-2125.2010.03715.x.
Li X, Shi L, Tang X, Wang Q, Zhou L, Song W, et al. Mechanistic prediction of food effects for compound a tablet using PBPK model. Saudi J Biol Sci. 2017;24:603–9. https://doi.org/10.1016/j.sjbs.2017.01.032.
Zhang T, Heimbach T, Lin W, Zhang J, He H. Prospective predictions of human pharmacokinetics for eighteen compounds. J Pharm Sci. 2015;104:2795–806. https://doi.org/10.1002/jps.24373.
Strougo A, Yassen A, Krauwinkel W, Danhof M, Freijer J. A semiphysiological population model for prediction of the pharmacokinetics of drugs under liver and renal disease conditions. Drug Metab Dispos. 2011;39:1278–87. https://doi.org/10.1124/dmd.110.037838.
Diaz D, Ford KA, Hartley DP, Harstad EB, Cain GR, Achilles-Poon K, et al. Pharmacokinetic drivers of toxicity for basic molecules: strategy to lower pKa results in decreased tissue exposure and toxicity for a small molecule Met inhibitor. Toxicol Appl Pharmacol. 2013;266:86–94. https://doi.org/10.1016/j.taap.2012.10.026.
Klotz U, McHorse T, Wilkinson G, Schenker S. The effect of cirrhosis on the disposition and elimination of meperidine in man. Clin Pharmacol Ther. 1974;16:667–75. https://doi.org/10.1002/cpt1974164667.
Sato Y, Miyashita A, Iwatsubo T, Usui T. Simultaneous absolute protein quantification of carboxylesterases 1 and 2 in human liver tissue fractions using liquid chromatography-tandem mass spectrometry. Drug Metab Dispos. 2012;40:1389–96. https://doi.org/10.1124/dmd.112.045054.
Yang D, Pearce RE, Wang X, Gaedigk R, Wan Y-JY, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–47. https://doi.org/10.1016/j.bcp.2008.10.005.
Crewe H, Barter Z, Rowland Yeo K, Rostami-Hodjegan A. Are there differences in the catalytic activity per unit enzyme of recombinantly expressed and human liver microsomal cytochrome P450 2C9 a systematic investigation into inter-system extrapolation factors. Biopharm Drug Dispos. 2011;32:303–18. https://doi.org/10.1002/bdd.760.
Proctor N, Tucker G, Rostami-Hodjegan A. Predicting drug clearance from recombinantly expressed CYPs: intersystem extrapolation factors. Xenobiotica. 2004;34:151–78. https://doi.org/10.1080/00498250310001646353.
Zhu H-J, Patrick KS, Yuan H-J, Wang J-S, Donovan JL, DeVane CL, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–8. https://doi.org/10.1016/j.ajhg.2008.04.015.
Shi J, Wang X, Eyler RF, Liang Y, Liu L, Mueller BA, et al. Association of oseltamivir activation with gender and carboxylesterase 1 genetic polymorphisms. Basic Clin Pharmacol Toxicol. 2016;119:555–61. https://doi.org/10.1111/bcpt.12625.
Wattanagoon Y, Stepniewska K, Lindegårdh N, Pukrittayakamee S, Silachamroon U, Piyaphanee W, et al. Pharmacokinetics of high-dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother. 2009;53:945–52. https://doi.org/10.1128/AAC.00588-08.
Sheu TG, Garten R, Smith C, Barnes J, Myrick A, Hillman M, et al. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. Morb Mortal Wkly Rep. 2009;58:1–3.
Food and Drug Administration.The medical direction of Tamiflu.2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021087s070lbl,%20021246s053lbl.pdf. Accessed 2 Oct 2019.
Wooltorton E. Oseltamivir (Tamiflu) unsafe in infants under 1 year old. Cmaj. 2004;170:336.
Funding
This work was supported by “Fujian Natural Science Fund (NO.2017J01188)”.
Author information
Authors and Affiliations
Contributions
Cuihong Lin designed the study and drafted the manuscript. Yong Chen, Meng Ke discussed the study and drafted the manuscript. Yong Chen, Meng Ke, Jianwen Xu performed the study. Yong Chen participated in data analysis, Meng Ke revised the manuscript.
The authors declare that they had no conflicts of interests in their authorship or publication of this contribution.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Ke, M., Xu, J. et al. Simulation of the Pharmacokinetics of Oseltamivir and Its Active Metabolite in Normal Populations and Patients with Hepatic Cirrhosis Using Physiologically Based Pharmacokinetic Modeling. AAPS PharmSciTech 21, 98 (2020). https://doi.org/10.1208/s12249-020-1638-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-1638-y