Abstract
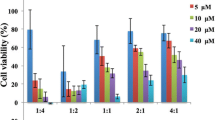
The purpose of this research is to develop a liposomal drug delivery system, which can selectively target hepatocellular carcinoma (HCC) to deliver the antitumor agent N-14NCTDA, a C14 alkyl chain norcantharimide derivative of norcantharidin. N-14NCTDA-loaded liposomes were successfully prepared by lipid membrane hydration and extrusion methods. SP94, a targeting peptide for HCC cells, was attached to the liposomes loaded with N-14NCTDA by the post-insertion method to obtain SP94 modified liposomes (SP94-LPs). SP94-LPs had a significant cytotoxicity against Hep G2 cells with the IC50 of 15.395 ± 0.89 μg/mL, which is lower than that of NCTD-S (IC50 = 20.863 ± 0.56 μg/mL) and GAL-LPs (IC50 = 24.589 ± 1.02 μg/mL). Compared with conventional liposomes (Con-LPs), SP94-LPs showed greater cellular uptake in Hep G2 cells. Likewise, significant tumor suppression was achieved in H22 tumor-bearing mice which were treated with SP94-LPs. The tumor inhibition rate (IRw) of SP94-LPs was 82 ± 0.98%, obviously higher than that of GAL-LPs (69 ± 1.39%), Con-LPs (60 ± 2.78%), and NCTD-S (51 ± 3.67%). SP94-LPs exhibited a significant hepatocellular carcinoma-targeting activity in vitro and in vivo, which will provide a new alternative for hepatocellular carcinoma treatment in future.

Graphical Abstract









Similar content being viewed by others
References
Chen M, Zhou X, Chen R, Wang J, Ye RD, Wang Y, et al. Nano-carriers for delivery and targeting of active ingredients of Chinese medicine for hepatocellular carcinoma therapy. Mater Today. 2019;25:66-87.
Bei YY, Chen XY, Liu Y, Xu JY, Wang WJ, Gu ZL, et al. Novel norcantharidin-loaded liver targeting chitosan nanoparticles to enhance intestinal absorption. Int J Nanomed. 2012;7:1819–27.
Anna PS, Agnieszka SC. New norcantharidin analogs: synthesis and anticancer activity. Arch Pharm. 2015;348(12):897–907.
Thaqi A, Scott JL, Gilbert J, Sakoff JA, Mccluskey A. Synthesis and biological activity of Delta-5,6-norcantharimides: importance of the 5,6-bridge. Eur J Med Chem. 2010;45(5):1717–23.
Xu X, Li Y, Wang F, Lv L, Liu J, Li M, et al. Synthesis, in vitro and in vivo evaluation of new norcantharidin-conjugated hydroxypropyltrimethyl ammonium chloride chitosan derivatives as polymer therapeutics. Int J Pharm. 2013;453(2):610–9.
Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6(2):263–5.
Yang P-Y, Chen M-F, Kao Y-H, Hu D-N, Chang F-R, Wu Y-C. Norcantharidin induces apoptosis of breast cancer cells: involvement of activities of mitogen activated protein kinases and signal transducers and activators of transcription. Toxicol in Vitro. 2011;25(3):699–707.
Wang Q, Zhang L, Hu W, Hu Z-H, Bei Y-Y, Xu J-Y, et al. Norcantharidin-associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine. 2010;6(2):371–81.
Lu K, Cao M, Mao W, Sun X, Tang J, Shen Y, et al. Targeted acid-labile conjugates of norcantharidin for cancer chemotherapy. J Mater Chem. 2012;22(31):15804–11.
Wang S, Guo S. Disodium norcantharidate-loaded poly(ɛ-caprolactone) microspheres: II. Modification of morphology and release behavior. Int J Pharm. 2008;353(1–2):15–20.
Zhang J, Tang Y, Li S, Liao C, Guo X. Targeting of the B-lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J Drug Target. 2010;18(9):675–87.
Liu X, Heng WS, Paul, Li Q, Chan LW. Novel polymeric microspheres containing norcantharidin for chemoembolization. J Control Release 2006;116(1):35–41.
Zhou D-h, Zhang J, Zhang G, Gan Z-h. Effect of surface charge of polymeric micelles on in vitro cellular uptake. Chin J Polym Sci. 2013;31(9):1299–309.
Ma J, Teng H, Wang J, Zhang Y, Ren T, Tang X, et al. A highly stable norcantharidin loaded lipid microspheres: preparation, biodistribution and targeting evaluation. Int J Pharm. 2014;473(1–2):475–84.
Hill TA, Stewart SG, Ackland SP, Gilbert J, Sauer B, Sakoff JA, et al. Norcantharimides, synthesis and anticancer activity: synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg Med Chem. 2007;15(18):6126–34.
Pan Z, Niu Y, Wang Y, Tang Y, Tang X, Cai C. Intravenous lipid microspheres loaded with alkylated norcantharidin derivative norcantharimide: improved stability and prolonged half-life. Eur J Lipid Sci Technol. 2015;117(1):55–64.
Bosslet K, Straub R, Blumrich M, Czech J, Gerken M, Sperker B, et al. Elucidation of the mechanism enabling tumor selective prodrug monotherapy. Cancer Res. 1998;58(6):1195–201.
Guan M, Zhou Y, Zhu QL, Liu Y, Bei YY, Zhang XN, et al. N-trimethyl chitosan nanoparticle-encapsulated lactosyl-norcantharidin for liver cancer therapy with high targeting efficacy. Nanomed Nanotechnol Biol Med. 2012;8(7):1172–81.
Narahara RTMMSTKAS. Development of human hepatocellular carcinoma cell-targeted protein cages. Bioconjug Chem. 2012;3(7):1494–501.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–7.
Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71.
Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57.
Matosevic CZS. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Delivery. 2016;1.
Wang J-Y, Chen J, Yang J, Wang H, Shen X, Sun Y-M, et al. Effects of surface charges of gold nanoclusters on long-term in vivo biodistribution, toxicity, and cancer radiation therapy. Int J Nanomedicine. 2016;11:3475–85.
Zhang YQ, Shen Y, Liao MM, Mao X, Mi GJ, You C, et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine. 2019;15(1):86–97.
Hu W, Chen Z, Wang M, Luo T, Wang J. Design, synthesis and evaluation of HepDirect fluorescence probes mediated by asialoglycoprotein receptor. Dyes Pigments. 2018;159:471–8.
Zhang X, Ng HLH, Lu A, Lin C, Zhou L, Lin G, et al. Drug delivery system targeting advanced hepatocellular carcinoma: current and future. Nanomed Nanotechnol Biol Med. 2016;12(4):853–69.
Lo A, Lin C-T, Wu H-C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther. 2008;7(3):579–89.
Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, et al. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano. 2011;5(7):5729–45.
Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10(5):389–97.
Medina SH, Tiruchinapally G, Chevliakov MV, Durmaz YY, Stender RN, Ensminger WD, et al. Targeting hepatic cancer cells with pegylated dendrimers displaying N-acetylgalactosamine and SP94 peptide ligands. Adv Healthc Mater. 2013;2(10):1337–50.
Liu X, Han M, Xu J, Geng S, Zhang Y, Ye X, et al. Asialoglycoprotein receptor-targeted liposomes loaded with a norcantharimide derivative for hepatocyte-selective targeting. Int J Pharm. 2017;520(1–2):98–110.
Wang DD, Wang T, Zhao GC, Zhuang J, Wu W. Improving systemic circulation of paclitaxel nanocrystals by surface hybridization of DSPE-PEG2000. Colloid Surf B-Biointerfaces. 2019;182:8.
Weng W, Wang Q, Wei C, Man N, Zhang K, Wei Q, et al. Preparation, characterization, pharmacokinetics and anti-hyperuricemia activity studies of myricitrin-loaded proliposomes. Int J Pharm. 2019;572:118735.
Bai J, Liu Z, Liu J, Zhang S, Tian Y, Zhang Y, et al. Mitochondrial metabolic study guided by proteomics analysis in hepatocellular carcinoma cells surviving long-term incubation with the highest dose of sorafenib. Aging-Us. 2019;11(24):12452–75.
Silva AM, Alvarado HL, Abrego G, Martins-Gomes C, Garduno-Ramirez ML, Garcia ML, et al. In vitro cytotoxicity of oleanolic/ursolic acids-loaded in PLGA nanoparticles in different cell lines. Pharmaceutics. 2019;11(8).
Zheng JS, Wang B, Jin YZ, Weng B, Chen JC. Nanostructured MXene-based biomimetic enzymes for amperometric detection of superoxide anions from HepG2 cells. Microchim Acta. 2019;186(2):9.
Yang F, Li J, Zhu J, Wang D, Chen S, Bai X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling pathway in H22 tumor-bearing mice. Eur J Pharmacol. 2015;754:105–14.
Olson F, Hunt CA, Szoka FC, Vail WJ, Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta Biomembr. 1979;557(1):9–23.
Wei MY, Xu YH, Zou Q, Tu LX, Tang CY, Xu T, et al. Hepatocellular carcinoma targeting effect of PEGylated liposomes modified with lactoferrin. Eur J Pharm Sci. 2012;46(3):131–41.
Wu Y, Zhang Y, Dai LL, Wang QQ, Xue LJ, Su ZG, et al. An apoptotic body-biomimic liposome in situ upregulates anti-inflammatory macrophages for stabilization of atherosclerotic plaques. J Control Release. 2019;316:236–49.
Jingying Z, Yongmin T, Sisi L, Chan L, Xiaoping G. Targeting of the B-lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J Drug Target. 2010;18(9):675–87.
Lin X, Zhang B, Zhang K, Zhang Y, Wang J, Qi N, et al. Preclinical evaluations of norcantharidin-loaded intravenous lipid microspheres with low toxicity. Expert Opin Drug Deliv. 2012;9(12):1449–62.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6(5):1949–57.
Felber AE, Dufresne MH, Leroux JC. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev. 2012;64(11):979–92.
Mali AD, Bathe RS. An updated review on liposome drug delivery system 2015;5(3):151.
Min J, Moon H, Yang HJ, Shin HH, Hong SY, Kang S. Development of P22 viral capsid nanocomposites as anti-cancer drug, bortezomib (BTZ), delivery nanoplatforms. Macromol Biosci. 2014;14(4):557–64.
Li DC, Zhong XK, Zeng ZP, Jiang JG, Li L, Zhao MM, et al. Application of targeted drug delivery system in Chinese medicine. J Control Release. 2009;138(2):103–12.
Liu MC, Liu L, Wang XR, Shuai WP, Hu Y, Han M, et al. Folate receptor-targeted liposomes loaded with a diacid metabolite of norcantharidin enhance antitumor potency for H22 hepatocellular carcinoma both in vitro and in vivo. Int J Nanomed. 2016;11:1395-412.
Funding
This study received financial support from the Liaoning Province Natural Science Fund Project (No.20180551031), and Overseas returnees start-up fund of Shenyang Pharmaceutical University (No. GGJJ2018102).
Author information
Authors and Affiliations
Contributions
YJ and XLL performed all experiments and wrote the manuscript. YXT contributed to data acquisition and analysis. Others contributed to data acquisition. The corresponding author YZ contributed to conception, design, data interpretation, and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Y., Liu, X., Tan, X. et al. In Vitro and In Vivo Evaluation of SP94 Modified Liposomes Loaded with N-14NCTDA, a Norcantharimide Derivative for Hepatocellular Carcinoma-Targeting. AAPS PharmSciTech 21, 277 (2020). https://doi.org/10.1208/s12249-020-01829-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01829-3