Abstract
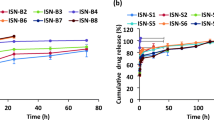
In order to tackle the problems on low water solubility of teniposide, involvement of toxic surfactant in its injection, and the poor stability during infusion, a Cremophor-free teniposide self-microemulsified drug delivery system (TEN-SMEDDS) was prepared for the first time, characterized, and evaluated in comparison with teniposide injection (VUMON) in vitro and in vivo. The optimized formulation contained N, N-dimethylacetamide, medium-chain triglyceride, lecithin, and dehydrated alcohol besides teniposide. The TEN-SMEDDS could form fine droplets with mean diameter of 282 ± 21 nm and zeta potential of −7.5 ± 1.7 mV after dilution with 5% glucose, which were stable within 4 h. The release of teniposide from TEN-SMEDDS and VUMON was similar. However, the pharmacokinetic behavior of TEN-SMEDDS in rats was different from that of VUMON, evidenced by the lower area under the concentration–time curve and larger volume of distribution in emulsion group. Finally, TEN-SMEDDS was found to distribute more teniposide in most tissues, especially in reticuloendothelial system, after intravenous administration to rats. Importantly, brain drug level in TEN-SMEDDS group was higher than or similar to that in control group, although the emulsion system had a lower plasma drug concentration. In conclusion, the novel SMEDDS prepared here, without toxic surfactant and as an oil solution before use, may be potential for clinical use due to its low toxicity and high store stability. It may be favorable for the treatment of some tumors like cerebroma, since it may achieve the relatively higher drug level in brain but lower blood concentration.





Similar content being viewed by others
REFERENCES
McCowage GB, Vowels MR, Shaw PJ, Lockwood L, Mameghan H. Autologous bone marrow transplantation for advanced neuroblastoma using teniposide, doxorubicin, melphalan, cisplatin, and total-body irradiation. J Clin Oncol. 1995;13(11):2789–95.
Hayes FA, Abromowitch M, Green AA. Allergic reactions to teniposide in patients with neuroblastoma and lymphoid malignancies. Cancer Treat Rep. 1985;69(4):439–41.
Ruskone-Fourmestraux A, Delmer A, Lavergne A, Molina T, Brousse N, Audouin J, et al. Multiple lymphomatous polyposis of the gastrointestinal tract: prospective clinicopathologic study of 31 cases. Groupe D’etude des Lymphomes Digestifs. Gastroenterology. 1997;112(1):7–16.
Wang J, Cui Y, Tang X. Chemical stability of teniposide in aqueous and parenteral lipid emulsions. Drug Dev Ind Pharm. 2009;35(4):508–13.
Carstensen H, Nolte H, Hertz H. Teniposide-induced hypersensitivity reactions in children. Lancet. 1989;2(8653):55.
Nolte H, Carstensen H, Hertz H. VM-26 (teniposide)-induced hypersensitivity and degranulation of basophils in children. Am J Pediatr Hematol Oncol. 1988;10(4):308–12.
Shimizu H, Frankel LS, Culbert SJ. Severe hypertensive reactions to teniposide (VM-26) in infants with congenital leukemia. Am J Pediatr Hematol Oncol. 1987;9(3):239–41.
Schwartsmann G, Sprinz E, Kronfeld M, Vinholes J, Sander E, Zampese M, et al. Phase II study of teniposide in patients with AIDS-related Kaposi's sarcoma. Eur J Cancer. 1991;27(12):1637–9.
Weller M, Müller B, Koch R, Bamberg M, Krauseneck P. Neuro-Oncology Working Group 01 trial of nimustine plus teniposide versus nimustine plus cytarabine chemotherapy in addition to involved-field radiotherapy in the first-line treatment of malignant glioma. J Clin Oncol. 2003;21(17):3276–84.
Mañé JM, Fernández R, Muñoz A, Rubio I, Ferreiro J, López-Argumedo G, et al. Preradiation chemotherapy with VM-26 and CCNU in patients with glioblastoma multiforme. Tumori. 2004;90(6):562–6.
Gelderblom H, Verweii J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8.
Kubisz P, Seghier F, Dobrotora M, Stasko J. Influence of teniposide on platelet functions in vitro. Thromb Res. 1995;77(2):145–8.
de Vries EG, Mulder NH, Postmus PE, Vriesendorp R, Willemse PH, Sleijfer DT. High-dose teniposide for refractory malignancies: a phase I study. Cancer Treat Rep. 1986;70(5):595–8.
Nornoo AO, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm. 2008;349(1–2):117–23.
Nornoo AO, Osborne DW, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel I: formulation, cytotoxicity and hemolysis. Int J Pharm. 2008;349(1–2):108–16.
Liliemark E, Sjöström B, Liliemark J, Peterson C, Kållberg N, Larsson BS. Targeting of teniposide to the mononuclear phagocytic system (MPS) by incorporation in liposomes and submicron lipid particles; an autoradiographic study in mice. Leuk Lymphoma. 1995;18(1–2):113–8.
Gan L, Gan Y, Zhu C, Zhang X, Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm. 2009;365(1–2):143–9.
Yin YM, Cui FD, Mu CF, Choi MK, Kim JS, Chung SJ, et al. Docetaxel microemulsion for enhanced oral bioavailability: preparation and in vitro and in vivo evaluation. J Control Release. 2009;140(2):86–94.
Piao HM, Balakrishnan P, Cho HJ, Kim H, Kim YS, et al. Preparation and evaluation of fexofenadine microemulsion for intranasal delivery. Int J Pharm. 2010;395(1–2):309–16.
Jogani VV, Shah PJ, Mishra P, Mishra AK, Mishra AR. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008;22(2):116–24.
Li X, Yue Y, Zhou Y, Fan Y, Fan C, Huang Y, et al. An oil-free microemulsion for intravenous delivery of diallyl trisulfide: formulation and evaluation. Int J Pharm. 2011;407(1–2):158–66.
Darole PS, Hegde DD, Nair HA. Formulation and evaluation of microemulsion based delivery system for amphotericin B. AAPS PharmSciTech. 2008;9(1):122–8.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Cao Y, Ni X, Sheng J. Comparison of microstructures of microemulsion and swollen micelle in electrokinetic chromatography. J Chromatogr A. 2011;1218(18):2598–603.
Mrestani Y, Behbood L, Albert H, Neubert RHH. Microemulsion and mixed micelle for oral administration as new drug formulations for highly hydrophilic drugs. Eur J Pharm Biopharm. 2010;74(2):219–22.
Narang AS, Delmarre D, Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007;345(1–2):9–25.
Nagai N, Shikii T, Mihara K, Ogata H, Sasaki Y. Improved high-performance liquid chromatographic analysis of teniposide in human plasma. J Chromatogr B: Biomed Sci Appl. 1998;709(2):315–9.
Sharma G, Wilson K, Walle CF, Sattar N, Petrie JR, Kumar R. Microemulsions for oral delivery of insulin: design, development and evaluation in streptozotocin induced diabetic rats. Eur J Pharm Biopharm. 2010;76(2):159–69.
Liu J, Gong T, Wang CG, Zhong ZR, Zhang ZR. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007;340(1–2):153–62.
Ganta S, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in parenteral emulsion. Int J Pharm. 2008;360(1–2):115–21.
Kim SJ, Choi HK, Suh SP, Lee YB. Pharmacokinetic and pharmacodynamic evaluation of cyclosporin A O/W-emulsion and microsphere formulations in rabbits. Eur J Pharm Sci. 2002;15(5):497–502.
Lu Y, Zhang Y, Yang ZY, Tang X. Formulation of an intravenous emulsion loaded with a clarithromycin–phospholipid complex and its pharmacokinetics in rats. Int J Pharm. 2009;366(1–2):160–9.
Shi S, Chen H, Lin X, Tang X. Pharmacokinetics, tissue distribution and safety of cinnarizine delivered in lipid emulsion. Int J Pharm. 2010;383(1–2):264–70.
Henningsson A, Sparreboom A, Sandstrom M, Freijs A, Larsson R, Bergh J, et al. Population pharmacokinetic modelling of unbound and total plasma concentrations of paclitaxel in cancer patients. Eur J Cancer. 2003;39(8):1105–14.
Sykes E, Woodburn K, Decker D, Kessel D. Effects of Cremophor EL on distribution of Taxol to serum lipoproteins. Br J Cancer. 1994;70(3):401–4.
Sparreboom A, Zuylen L, Brouwer E, Loos WJ, Bruijn P, Gelderblom M, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59(7):1454–7.
Ma HL, Xu YF, Qi XR, Maitani Y, Nagai T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int J Pharm. 2008;354(1–2):217–26.
Oyewumi MO, Yokel RA, Jay M, Coakley T, Mumper RJ. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Control Release. 2004;95(3):613–26.
ACKNOWLEDGMENTS
This study was funded by the National Nature Science Foundation (no. 81130059), the National Basic Research Program of China (no. 2009CB930300), and Innovation Team of Ministry of Education (no. BMU20110263). The authors are grateful for the support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, S., Cui, Z., Mei, D. et al. A Cremophor-Free Self-Microemulsified Delivery System for Intravenous Injection of Teniposide: Evaluation In Vitro and In Vivo . AAPS PharmSciTech 13, 846–852 (2012). https://doi.org/10.1208/s12249-012-9809-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9809-0