Abstract
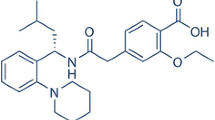
The effect of complexation of irbesartan (IRB), a practically water-insoluble drug, with cyclodextrins in presence of different concentrations of water-soluble polymers (PEG 4000 and PVP K-90) on the dissolution rate of the drug has been investigated. Phase solubility studies were carried out to evaluate the solubilizing power of βCD in association with water-soluble polymers towards IRB and to determine the apparent stability constant (K S) of the complexes. Improvement in K S value for ternary complexes (IRB–βCD–polymers) clearly proved the benefit on the addition of water-soluble polymer to increase complexation efficiency. The dissolution rate of the drug from ternary systems containing PEG 4000 and PVP K-90 was higher as compared to the binary system. An optimum increase in the dissolution rate of the drug was observed at a polymer concentration of 5% w/w for PVP K-90 and 10% w/w for PEG 4000. DSC, FTIR, SEM, and XRD studies were carried out to characterize the complexes.







Similar content being viewed by others
Abbreviations
- IRB:
-
irbesartan
- βCD:
-
beta-cyclodextrins
- DSC:
-
differential scanning calorimetry
- FTIR:
-
Fourier transform infrared spectroscopy
- XRD:
-
X-ray diffraction
- SEM:
-
scanning electron microscopy
- K S :
-
apparent stability constant
- CE:
-
co-evaporation
- HCl:
-
hydrochloric acid
References
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123:78–99.
Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilisers. Adv Drug Deliv Rev. 2007;59:645–66.
S.C. Sweetman. Martindale-The Complete Drug Reference, ed: 34, pg no 940. London, 2005.
Pouleur HG. Clinical overview of irbesartan a new angiotensin II receptor antagonist. Am J Hypertens. 1997;10:318–24.
Chawla G, Bansal AK. A comparative assessment of solubility advantage from glassy and crystalline forms of a water-insoluble drug. Eur J Pharm Sci. 2007;32:45–57.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins.1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–25.
Valero M, Perez-Revuelta BI, Rodriguez LJ. Effect of PVP K-25 on the formation of the naproxen:β-cyclodextrin complex. Int J Pharm. 2003;253:97–110.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;06:E329–57.
Higuchi T, Connors KA. Phase solubility diagram. Adv Anal Chem Instr. 1965;4:117–212.
Ribeiro LSS, Ferreira DC, Veiga FJB. Physicochemical investigation of the effects of water-soluble polymers on vinpocetine complexation with β-cyclodextrin and its sulfobutyl ether derivative in solution and solid state. Eur J Pharm Sci. 2003;20:253–66.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. Int J Pharm. 2006;309:129–38.
Liu L, Zhu S. Preparation and characterization of inclusion complexes of Prazosin hydrochloride with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J Pharm Biomed Anal. 2006;40:122–7.
Patel AR, Vavia PR. Effect of hydrophilic polymer on solubilisation of fenofibrate by cyclodextrin complexation. J Incl Phenom Macroc Chem. 2006;56:247–51.
Diaz MTE, Mfndez MG, Pfrez-Marcos MB, Vila-Jato JL, Torres-Labandeira JJ. Characterization and in vitro dissolution behaviour of ketoconazole/β-and 2-hydroxypropyl-β-cyclodextrin inclusion compounds. Int J Pharm. 1996;143:203–10.
Figueiras A, Ribeiro L, Veira MT. Preparation and characterization of omeprazole:methyl–beta-cyclodextrininclusion complex in solid state. J Incl Phenom Macroc Chem. 2007;57:173–7.
Naidu NB, Chowdary KPR, Murthy KVR, Satyanarayana V, Hayman AR, Becket G. Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary systems. J Pharm Biomed Anal. 2004;35:75–86.
Ammar HO, Salama HA, Mahmoud AA. Implication of inclusion complexation of glimepiride in cyclodextrin–polymer systems on its dissolution, stability and therapeutic efficacy. Int J Pharm. 2006;320:53–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirlekar, R.S., Sonawane, S.N. & Kadam, V.J. Studies on the Effect of Water-Soluble Polymers on Drug–Cyclodextrin Complex Solubility. AAPS PharmSciTech 10, 858–863 (2009). https://doi.org/10.1208/s12249-009-9274-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9274-6