Abstract
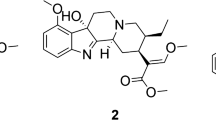
Speciociliatine, a diastereomer of mitragynine, is an indole-based alkaloid found in kratom (Mitragyna speciosa). Kratom has been widely used for the mitigation of pain and opioid dependence, as a mood enhancer, and/or as an energy booster. Speciociliatine is a partial µ-opioid agonist with a 3-fold higher binding affinity than mitragynine. Speciociliatine has been found to be a major circulating alkaloid in humans following oral administration of a kratom product. In this report, we have characterized the metabolism of speciociliatine in human and preclinical species (mouse, rat, dog, and cynomolgus monkey) liver microsomes and hepatocytes. Speciociliatine metabolized rapidly in monkey, rat, and mouse hepatocytes (in vitro half-life was 6.6 ± 0.2, 8.3 ± 1.1, 11.2 ± 0.7 min, respectively), while a slower metabolism was observed in human and dog hepatocytes (91.7 ± 12.8 and > 120 min, respectively). Speciociliatine underwent extensive metabolism, primarily through monooxidation and O-demethylation metabolic pathways in liver microsomes and hepatocytes across species. No human-specific or disproportionate metabolites of speciociliatine were found in human liver microsomes. The metabolism of speciociliatine was predominantly mediated by CYP3A4 with minor contributions by CYP2D6.






Similar content being viewed by others
References
Adkins JE, Boyer EW, McCurdy CR. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem. 2011;11(9):1165–75. https://doi.org/10.2174/156802611795371305.
Berthold EC, Kamble SH, Raju KS, King TI, Popa R, Sharma A, et al. Preclinical pharmacokinetic study of speciociliatine, a kratom alkaloid, in rats using an UPLC-MS/MS method. J Pharm Biomed Anal. 2021;194: 113778. https://doi.org/10.1016/j.jpba.2020.113778.
Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement, kratom. Am J Addict. 2007;16(5):352–6. https://doi.org/10.1080/10550490701525368.
Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, et al. Following “the roots” of kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in western countries. Biomed Res Int. 2015;2015: 968786. https://doi.org/10.1155/2015/968786.
Kruegel AC, Grundmann O. The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology. 2018;134(Pt A):108–20. https://doi.org/10.1016/j.neuropharm.2017.08.026.
Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, et al. 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992–1001. https://doi.org/10.1021/acscentsci.9b00141.
Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916–28. https://doi.org/10.1248/cpb.52.916
Ellis CR, Racz R, Kruhlak NL, Kim MT, Zakharov AV, Southall N, et al. Evaluating kratom alkaloids using PHASE. PLoS ONE. 2020;15(3): e0229646. https://doi.org/10.1371/journal.pone.0229646.
Todd DA, Kellogg JJ, Wallace ED, Khin M, Flores-Bocanegra L, Tanna RS, et al. Chemical composition and biological effects of kratom (Mitragyna speciosa): in vitro studies with implications for efficacy and drug interactions. Sci Rep. 2020;10(1):19158. https://doi.org/10.1038/s41598-020-76119-w.
Sharma A, Kamble SH, Leon F, Chear NJ, King TI, Berthold EC, et al. Simultaneous quantification of ten key kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2019;11(8):1162–71. https://doi.org/10.1002/dta.2604.
Kamble SH, Sharma A, King TI, Leon F, McCurdy CR, Avery BA. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279–88. https://doi.org/10.1080/00498254.2018.1552819.
Tanna RS, Nguyen JT, Hadi DL, Manwill PK, Flores-Bocanegra L, Layton ME, et al. Clinical pharmacokinetic assessment of kratom (Mitragyna speciosa), a botanical product with opioid-like effects, in healthy adult participants. Pharmaceutics. 2022;14(3):620. https://doi.org/10.3390/pharmaceutics14030620
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, et al. From kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37(2):138–51. https://doi.org/10.1016/j.neubiorev.2012.11.012.
Kamble SH, Berthold EC, King TI, Raju Kanumuri SR, Popa R, Herting JR, et al. Pharmacokinetics of eleven kratom alkaloids following an oral dose of either traditional or commercial kratom products in rats. J Nat Prod. 2021;84(4):1104–12. https://doi.org/10.1021/acs.jnatprod.0c01163.
Singh D, Yeou Chear NJ, Narayanan S, Leon F, Sharma A, McCurdy CR, et al. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J Ethnopharmacol. 2020;249: 112462. https://doi.org/10.1016/j.jep.2019.112462.
Obeng S, Kamble SH, Reeves ME, Restrepo LF, Patel A, Behnke M, et al. Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J Med Chem. 2020;63(1):433–9. https://doi.org/10.1021/acs.jmedchem.9b01465.
Gutridge AM, Chakraborty S, Varga BR, Rhoda ES, French AR, Blaine AT, et al. Evaluation of kratom opioid derivatives as potential treatment option for alcohol use disorder. Front Pharmacol. 2021;12. https://doi.org/10.3389/fphar.2021.764885.
Damodaran T, Chear NJ-Y, Murugaiyah V, Mordi MN, Ramanathan S. Comparative toxicity assessment of kratom decoction, mitragynine and speciociliatine versus morphine on zebrafish (Danio rerio) embryos. Front Pharmacol. 2021:2193. https://doi.org/10.3389/fphar.2021.714918.
Avery BA, Boddu SP, Sharma A, Furr EB, Leon F, Cutler SJ, et al. Comparative pharmacokinetics of mitragynine after oral administration of Mitragyna speciosa (kratom) leaf extracts in rats. Planta Med. 2019;85(4):340–6. https://doi.org/10.1055/a-0770-3683.
Philipp AA, Wissenbach DK, Weber AA, Zapp J, Maurer HH. Metabolism studies of the kratom alkaloid speciociliatine, a diastereomer of the main alkaloid mitragynine, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. Anal Bioanal Chem. 2011;399(8):2747–53. https://doi.org/10.1007/s00216-011-4660-9.
Patil PG, Kamble SH, Shah TS, Iyer KR. Effect of water miscible organic solvents on p-nitrophenol hydroxylase (cyp2e1) activity in rat liver microsomes. Indian J Pharm Sci. 2015;77(3):283–9. https://doi.org/10.4103/0250-474x.159613.
Shah TS, Kamble SH, Patil PG, Iyer KR. Effect of water-miscible organic solvents on cyp450-mediated metoprolol and imipramine metabolism in rat liver microsomes. Indian J Pharm Sci. 2015;77(4):382–90. https://doi.org/10.4103/0250-474x.164783.
Ahire D, Sinha S, Brock B, Iyer R, Mandlekar S, Subramanian M. Metabolite identification, reaction phenotyping, and retrospective drug-drug interaction predictions of 17-deacetylnorgestimate, the active component of the oral contraceptive norgestimate. Drug Metab Dispos. 2017;45(6):676–85. https://doi.org/10.1124/dmd.116.073940.
Walsky RL, Obach RS. A comparison of 2-phenyl-2-(1-piperidinyl)propane (ppp), 1,1’,1’’-phosphinothioylidynetrisaziridine (thioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab Dispos. 2007;35(11):2053–9. https://doi.org/10.1124/dmd.107.015883.
Yang X, Atkinson K, Di L. Novel cytochrome p450 reaction phenotyping for low-clearance compounds using the hepatocyte relay method. Drug Metab Dispos. 2016;44(3):460–5. https://doi.org/10.1124/dmd.115.067876.
Youdim KA, Zayed A, Dickins M, Phipps A, Griffiths M, Darekar A, et al. Application of CYP3A4 in vitro data to predict clinical drug-drug interactions; predictions of compounds as objects of interaction. Br J Clin Pharmacol. 2008;65(5):680–92. https://doi.org/10.1111/j.1365-2125.2007.03070.x.
Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8(1):33–45. https://doi.org/10.2174/138920007779315053.
Sohlenius-Sternbeck AK, Afzelius L, Prusis P, Neelissen J, Hoogstraate J, Johansson J, et al. Evaluation of the human prediction of clearance from hepatocyte and microsome intrinsic clearance for 52 drug compounds. Xenobiotica. 2010;40(9):637–49. https://doi.org/10.3109/00498254.2010.500407.
Ramanathan R, Su AD, Alvarez N, Blumenkrantz N, Chowdhury SK, Alton K, et al. Liquid chromatography/mass spectrometry methods for distinguishing N-oxides from hydroxylated compounds. Anal Chem. 2000;72(6):1352–9. https://doi.org/10.1021/ac9911692.
Patil A, Ladumor MK, Kamble SH, Johnson BM, Subramanian M, Sinz MW, et al. Identification of novel glutathione conjugates of terbinafine in liver microsomes and hepatocytes across species. Xenobiotica. 2019;49(12):1403–13. https://doi.org/10.1080/00498254.2019.1581959.
Zhuo X, Huang XS, Degnan AP, Snyder LB, Yang F, Huang H, et al. Identification of glutathione conjugates of acetylene-containing positive allosteric modulators of metabotropic glutamate receptor subtype 5. Drug Metab Dispos. 2015;43(4):578–89. https://doi.org/10.1124/dmd.114.061879.
Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47(9):1469–79. https://doi.org/10.1016/0006-2952(94)90520-7.
Kitajima M, Misawa K, Kogure N, Said IM, Horie S, Hatori Y, et al. A new indole alkaloid, 7-hydroxyspeciociliatine, from the fruits of Malaysian Mitragyna speciosa and its opioid agonistic activity. J Nat Med. 2006;60(1):28–35. https://doi.org/10.1007/s11418-005-0001-7.
Tanna RS, Tian DD, Cech NB, Oberlies NH, Rettie AE, Thummel KE, et al. Refined prediction of pharmacokinetic kratom-drug interactions: time-dependent inhibition considerations. J Pharmacol Exp Ther. 2021;376(1):64–73. https://doi.org/10.1124/jpet.120.000270.
Rowland M, Matin SB. Kinetics of drug-drug interactions. J Pharmacokinet Biopharm. 1973;1(6):553–67. https://doi.org/10.1007/BF01059791.
Funding
This study was supported by R01 DA047855 and UG3/UH3 DA048353 grants from the National Institute on Drug Abuse and the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427.
Author information
Authors and Affiliations
Contributions
SK, EB, KSRR, TK, MK: conceptualization, methodology, investigation, data curation, writing — original draft, preparation. MM, FL: methodology, investigation, writing — review & editing. LM, CRM: conceptualization, funding acquisition, supervision, writing — review & editing. AS: conceptualization, methodology, investigation, supervision, writing — original draft, preparation, review & editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamble, S.H., Berthold, E.C., Kanumuri, S.R.R. et al. Metabolism of Speciociliatine, an Overlooked Kratom Alkaloid for its Potential Pharmacological Effects. AAPS J 24, 86 (2022). https://doi.org/10.1208/s12248-022-00736-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-022-00736-8