Abstract
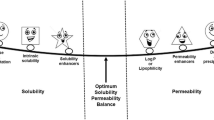
While each of the two key parameters of oral drug absorption, the solubility and the permeability, has been comprehensively studied separately, the relationship and interplay between the two have been largely ignored. For instance, when formulating a low-solubility drug using various solubilization techniques: what are we doing to the apparent permeability when we increase the solubility? Permeability is equal to the drug’s diffusion coefficient through the membrane times the membrane/aqueous partition coefficient divided by the membrane thickness. The direct correlation between the intestinal permeability and the membrane/aqueous partitioning, which in turn is dependent on the drug’s apparent solubility in the GI milieu, suggests that the solubility and the permeability are closely associated, exhibiting a certain interplay between them, and the current view of treating the one irrespectively of the other may not be sufficient. In this paper, we describe the research that has been done thus far, and present new data, to shed light on this solubility–permeability interplay. It has been shown that decreased apparent permeability accompanies the solubility increase when using different solubilization methods. Overall, the weight of the evidence indicates that the solubility–permeability interplay cannot be ignored when using solubility-enabling formulations; looking solely at the solubility enhancement that the formulation enables may be misleading with regards to predicting the resulting absorption, and hence, the solubility–permeability interplay must be taken into account to strike the optimal solubility–permeability balance, in order to maximize the overall absorption.








Similar content being viewed by others
References
Lennernas H. Human intestinal permeability. J Pharm Sci. 1998;87(4):403–10. doi:10.1021/js970332a.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19(3):359–76. doi:10.1016/0169-409x(96)00009-9.
Sun D, Yu LX, Hussain MA, Wall DA, Smith RL, Amidon GL. In vitro testing of drug absorption for drug ‘developability’ assessment: forming an interface between in vitro preclinical data and clinical outcome. Curr Opin Drug Discov Devel. 2004;7(1):75–85.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413.
Dahan A, Amidon GL. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharmaceutics. 2009;6(1):19–28. doi:10.1021/mp800088f.
Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–6. doi:10.1208/s12248-009-9144-x.
Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernas H, et al. High-permeability criterion for BCS classification: segmental/pH dependent permeability considerations. Mol Pharmaceutics. 2010;7(5):1827–34. doi:10.1021/mp100175a.
Lennernas H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49(7):627–38. doi:10.1111/j.2042-7158.1997.tb06084.x.
Lobenberg R, Amidon GL. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42(6):620–43.
Dahan A, Hoffman A. Use of a dynamic in vitro lipolysis model to rationalize oral formulation development for poor water soluble drugs: correlation with in vivo data and the relationship to intra-enterocyte processes in rats. Pharm Res. 2006;23(9):2165–74. doi:10.1007/s11095-006-9054-x.
Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129(1):1–10. doi:10.1016/j.jconrel.2008.03.021.
Gao Y, Carr RA, Spence JK, Wang WW, Turner TM, Lipari JM, et al. A pH-dilution method for estimation of biorelevant drug solubility along the gastrointestinal tract: application to physiologically based pharmacokinetic modeling. Mol Pharmaceutics. 2010;7(5):1516–26. doi:10.1021/mp100157s.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi:10.1016/s0169-409x(00)00129-0.
Stenberg P, Bergström CAS, Luthman K, Artursson P. Theoretical predictions of drug absorption in drug discovery and development. Clin Pharmacokinet. 2002;41(11):877–99.
Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59(7):645–66. doi:10.1016/j.addr.2007.05.012.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–25.
Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85(11):1142–69.
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78.
Loftsson T, Vogensen SB, Brewster ME, Konráðsdóttir F. Effects of cyclodextrins on drug delivery through biological membranes. J Pharm Sci. 2007;96(10):2532–46. doi:10.1002/jps.20992.
Loftsson T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2(2):335–51. doi:10.1517/17425247.2.1.335.
Loftsson T, Brewster ME, Masson M. Role of cyclodextrins in improving oral drug delivery. Am J Drug Deliv. 2004;2(4):261.
Rao VM, Stella VJ. When can cyclodextrins be considered for solubilization purposes? J Pharm Sci. 2003;92(5):927–32.
Miller LA, Carrier RL, Ahmed I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci. 2007;96(7):1691–707.
Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–49. doi:10.1002/jps.22033.
Gamsiz E, Miller L, Thombre A, Ahmed I, Carrier RL. Modeling the influence of cyclodextrins on oral absorption of low-solubility drugs: I. Model development. Biotech Bioeng. 2010;105(2):409–20.
Gamsiz E, Thombre A, Ahmed I, Carrier RL. Drug salts and solubilization: modeling the influence of cyclodextrins on oral absorption. Ann Biomed Eng. 2011;39(1):455–68. doi:10.1007/s10439-010-0169-1.
Gamsiz ED, Miller L, Thombre AG, Ahmed I, Carrier RL. Modeling the influence of cyclodextrins on oral absorption of low solubility drugs: II. Experimental validation. Biotechnol Bioeng. 2010;105(2):421–30. doi:10.1002/bit.22524.
Brewster ME, Noppe M, Peeters J, Loftsson T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int J Pharm. 2007;342(1–2):250–3. doi:10.1016/j.ijpharm.2007.04.029.
Amidon GE, Higuchi WI, Ho NFH. Theoretical and experimental studies of transport of micelle-solubilized solutes. J Pharm Sci. 1982;71(1):77–84. doi:10.1002/jps.2600710120.
Katneni K, Charman SA, Porter CJH. Permeability assessment of poorly water-soluble compounds under solubilizing conditions: the reciprocal permeability approach. J Pharm Sci. 2006;95(10):2170–85. doi:10.1002/jps.20687.
Katneni K, Charman SA, Porter CJH. Impact of cremophor-EL and polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. 2007;96(2):280–93. doi:10.1002/jps.20779.
Katneni K, Charman SA, Porter CJH. Use of plasma proteins as solubilizing agents in in vitro permeability experiments: correction for unbound drug concentration using the reciprocal permeability approach. J Pharm Sci. 2008;97(1):209–24. doi:10.1002/jps.20877.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, Amidon GL, Dahan A. The solubility–permeability interplay: mechanistic modeling and predictive application of the impact of micellar solubilization on intestinal permeation. Mol Pharmaceutics. 2011;8(5):1848–56. doi:10.1021/mp200181v.
Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa. 3: effects of polyethoxylated solubilizing agents on drug permeation and metabolism. J Pharm Sci. 2010;99(2):1016–27. doi:10.1002/jps.21836.
Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa: 1. Application of an in situ perfusion model to simultaneously assess drug permeation and metabolism. J Pharm Sci. 2010;99(2):982–98. doi:10.1002/jps.21912.
Mudra DR, Jin JY, Borchardt RT. Absorption barriers in the rat intestinal mucosa: 2. Application of physiologically based mathematical models to quantify mechanisms of drug permeation and metabolism. J Pharm Sci. 2010;99(2):999–1015. doi:10.1002/jps.21965.
Nerurkar MM, Ho NFH, Burton PS, Vidmar TJ, Borchardt RT. Mechanistic roles of neutral surfactants on concurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J Pharm Sci. 1997;86(7):813–21. doi:10.1021/js960483y.
Poelma FGJ, Breäs R, Tukker JJ. Intestinal absorption of drugs. III. The influence of taurocholate on the disappearance kinetics of hydrophilic and lipophilic drugs from the small intestine of the rat. Pharm Res. 1990;7(4):392–7. doi:10.1023/a:1015827624296.
Poelma FGJ, Breäs R, Tukker JJ, Crommelin DJA. Intestinal absorption of drugs. The influence of mixed micelles on the disappearance kinetics of drugs from the small intestine of the rat. J Pharm Pharmacol. 1991;43(5):317–24. doi:10.1111/j.2042-7158.1991.tb06697.x.
Yano K, Masaoka Y, Kataoka M, Sakuma S, Yamashita S. Mechanisms of membrane transport of poorly soluble drugs: role of micelles in oral absorption processes. J Pharm Sci. 2010;99(3):1336–45. doi:10.1002/jps.21919.
Buyukozturk F, Benneyan JC, Carrier RL. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Release. 2010;142(1):22–30. doi:10.1016/j.jconrel.2009.10.005.
Dahan A, Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67(1):96–105. doi:10.1016/j.ejpb.2007.01.017.
Giacomini K, Huang S, Tweedie D, Benet L, Brouwer K, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–36.
Riad LE, Sawchuk RJ. Effect of polyethylene glycol 400 on the intestinal permeability of carbamazepine in the rabbit. Pharm Res. 1991;8(4):491–7. doi:10.1023/a:1015803312233.
Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility–permeability interplay when using cosolvents for solubilization: revising the way we use solubility-enabling formulations. Mol Pharmaceutics. 2012. doi:10.1021/mp200460u.
Beig A, Miller JM, Dahan A. Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: the effect of PEG-400 on carbamazepine absorption. Eur J Pharm Biopharm. 2012. doi:10.1016/j.ejpb.2012.02.012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Ping Gao and Lawrence Yu
Rights and permissions
About this article
Cite this article
Dahan, A., Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J 14, 244–251 (2012). https://doi.org/10.1208/s12248-012-9337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9337-6