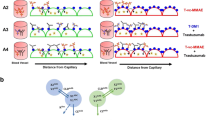
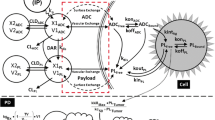
Abstract
Antibody–drug conjugates (ADCs) rely on high expression of target antigens on cancer cells to effectively enter the cell and release a cytotoxic payload. Previous studies have shown that ADC efficacy is not always tied to antigen expression. However, our recent in vitro study suggests a linear relationship between antigen expression and the intracellular levels of the ADC payload. In this study, we have explored the relationship between antigen expression and intratumoral ADC exposure in vivo. Using trastuzumab-vc-MMAE (T-vc-MMAE) and four cell lines with varying expression of human epithelial growth factor receptor 2 (HER2), the pharmacokinetics of total trastuzumab, released (“free”) MMAE, and total MMAE were evaluated in a tumor xenograft model. Nude mice were implanted with tumors originating from BT-474, MDA-MB-453, MCF-7, and MDA-MB-468 cell lines and dosed with 10 mg/kg or 1 mg/kg of ADC. Observed data were mathematically characterized using a mechanism-based PK model. A strong positive correlation was observed between antigen expression levels and free/total MMAE exposure (R2 ≥ 0.91) (total MMAE being the sum of released and conjugated MMAE) within the tumor, but not for total trastuzumab exposure. The PK model was able to recapitulate plasma PK through simulation; however, the tumor PK was overpredicted or underpredicted in some cases potentially due to differences in tumor vasculature or extracellular matrix conditions. Our results indicate a linear relationship between antigen expression and tumor exposure of free/total ADC payload in vivo, validating our previous finding in vitro, while also revealing the need to understand complex physiology of the tumor to predict tumor PK of ADC and its components. Our findings also support the concept of antigen expression screening in patients for targeted therapies like ADCs to achieve the maximum therapeutic benefit of the treatment.





Similar content being viewed by others
References
Chau CH, Steeg PS, Figg WD. Antibody–drug conjugates for cancer. Lancet. 2019;394(10200):793–804.
Collins DM, Bossenmaier B, Kollmorgen G, Niederfellner G. Acquired resistance to antibody-drug conjugates. Cancers (Basel). 2019;11(3).
Mullard A. 2019 FDA drug approvals. Nat Rev Drug Discov. 2020;19(2):79–84.
FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies [press release]. U.S. Food and Drug Administration, December 20, 2019.
FDA Approves New Therapy for Triple Negative Breast Cancer That Has Spread, Not Responded to Other Treatments [press release]. U.S. Food and Drug Administration, April 22, 2020.
Kalim M, Chen J, Wang S, Lin C, Ullah S, Liang K, et al. Intracellular trafficking of new anticancer therapeutics: antibody–drug conjugates. Drug Des Dev Ther. 2017;11:2265–76.
Bornstein GG. Antibody drug conjugates: preclinical considerations. AAPS J. 2015;17(3):525–34.
Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol. 2016;27(12):2168–72.
Lambert JM, Morris CQ. Antibody–drug conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Ther. 2017;34(5):1015–35.
Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209.
Li D, Poon KA, Yu SF, Dere R, Go M, Lau J, et al. DCDT2980S, an anti-CD22-monomethyl auristatin E antibody–drug conjugate, is a potential treatment for non-Hodgkin lymphoma. Mol Cancer Ther. 2013;12(7):1255–65.
O'Brien C, Cavet G, Pandita A, Hu X, Haydu L, Mohan S, et al. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68(13):5380–9.
Sharma S, Li Z, Bussing D, Shah DK. Evaluation of quantitative relationship between target expression and ADC exposure inside cancer cells. Drug Metab Dispos. 2020.
Singh AP, Shah DK. Application of a PK-PD modeling and simulation-based strategy for clinical translation of antibody–drug conjugates: a case study with trastuzumab emtansine (T-DM1). AAPS J. 2017;19(4):1054–70.
Singh AP, Maass KF, Betts AM, Wittrup KD, Kulkarni C, King LE, et al. Evolution of antibody–drug conjugate tumor disposition model to predict preclinical tumor pharmacokinetics of trastuzumab-emtansine (T-DM1). AAPS J. 2016;18(4):861–75.
Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59.
Singh AP, Sharma S, Shah DK. Quantitative characterization of in vitro bystander effect of antibody–drug conjugates. J Pharmacokinet Pharmacodyn. 2016;43(6):567–82.
Li Y, Gu C, Gruenhagen J, Yehl P, Chetwyn NP, Medley CD. An enzymatic deconjugation method for the analysis of small molecule active drugs on antibody–drug conjugates. MAbs. 2016;8(4):698–705.
Shah DK, King LE, Han X, Wentland JA, Zhang Y, Lucas J, et al. A priori prediction of tumor payload concentrations: preclinical case study with an auristatin-based anti-5T4 antibody–drug conjugate. AAPS J. 2014;16(3):452–63.
Maass KF, Kulkarni C, Betts AM, Wittrup KD. Determination of cellular processing rates for a trastuzumab–maytansinoid antibody–drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. 2016;18(3):635–46.
D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. 2009.
Mathworks. SimBiology: User's Guide (R2018b). 2018.
Flores BE. A pragmatic view of accuracy measurement in forecasting. Omega. 1986;14(2):93–8.
Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody–drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19.
Martin LP, Konner JA, Moore KN, Seward SM, Matulonis UA, Perez RP, et al. Characterization of folate receptor alpha (FRalpha) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: a phase I expansion study of the FRalpha-targeting antibody–drug conjugate mirvetuximab soravtansine. Gynecol Oncol. 2017;147(2):402–7.
Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):398–405.
Polson AG, Ho WY, Ramakrishnan V. Investigational antibody–drug conjugates for hematological malignancies. Expert Opin Investig Drugs. 2011;20(1):75–85.
Tong XM, Feng L, Suthe SR, Weng TH, Hu CY, Liu YZ, et al. Therapeutic efficacy of a novel humanized antibody–drug conjugate recognizing plexin-semaphorin-integrin domain in the RON receptor for targeted cancer therapy. J Immunother Cancer. 2019;7(1):250.
Le Joncour V, Martins A, Puhka M, Isola J, Salmikangas M, Laakkonen P, et al. A novel anti-HER2 antibody-drug conjugate XMT-1522 for HER2-positive breast and gastric cancers resistant to trastuzumab emtansine. Mol Cancer Ther. 2019;18(10):1721–30.
Mosher R, Vogel C, Shaw P, Zielinski D, Boesler C, Mühleisen A, et al. Target expression/efficacy relationship of XMT-1522, a HER2-targeting antibody drug conjugate (ADC), in an unselected series of non-small cell lung cancer (NSCLC) primary human carcinoma xenografts. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; Dublin, Ireland; 2018.
McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging. 2009;36(1):81–93.
Harrell JC, Pfefferle AD, Zalles N, Prat A, Fan C, Khramtsov A, et al. Endothelial-like properties of claudin-low breast cancer cells promote tumor vascular permeability and metastasis. Clin Exp Metastasis. 2014;31(1):33–45.
Makawita S, Meric-Bernstam F. Antibody–drug conjugates: patient and treatment selection. Am Soc Clin Oncol Educ Book. 2020;40:1–10.
Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22(6):701–6.
Palm S, Enmon RM Jr, Matei C, Kolbert KS, Xu S, Zanzonico PB, et al. Pharmacokinetics and biodistribution of (86)Y-trastuzumab for (90)Y dosimetry in an ovarian carcinoma model: correlative MicroPET and MRI. J Nucl Med. 2003;44(7):1148–55.
Ammons WS, Bauer RJ, Horwitz AH, Chen ZJ, Bautista E, Ruan HH, et al. In vitro and in vivo pharmacology and pharmacokinetics of a human engineered monoclonal antibody to epithelial cell adhesion molecule. Neoplasia. 2003;5(2):146–54.
Li F, Ulrich ML, Shih VF, Cochran JH, Hunter JH, Westendorf L, et al. Mouse strains influence clearance and efficacy of antibody and antibody–drug conjugate via Fc-FcgammaR interaction. Mol Cancer Ther. 2019;18(4):780–7.
Kurebayashi J, Otsuki T, Kunisue H, Mikami Y, Tanaka K, Yamamoto S, et al. Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res. 1999;90(9):977–81.
Farooqi AA, Siddik ZH. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem Funct. 2015;33(5):257–65.
Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002;73(3):237–43.
Chen R, Hou J, Newman E, Kim Y, Donohue C, Liu X, et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol Cancer Ther. 2015;14(6):1376–84.
Williams M, Spreafico A, Vashisht K, Hinrichs MJ. Patient selection strategies to maximize therapeutic index of antibody–drug conjugates: prior approaches and future directions. Mol Cancer Ther. 2020;19(9):1770–83.
Acknowledgments
This project was funded by the Center for Protein Therapeutics (CPT) at the University at Buffalo (UB). D.K.S. is supported by NIH grants GM114179, AI138195, and R01CA246785.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1021 kb)
Rights and permissions
About this article
Cite this article
Bussing, D., Sharma, S., Li, Z. et al. Quantitative Evaluation of the Effect of Antigen Expression Level on Antibody–Drug Conjugate Exposure in Solid Tumor. AAPS J 23, 56 (2021). https://doi.org/10.1208/s12248-021-00584-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00584-y