Abstract
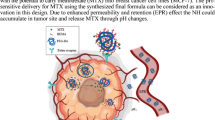
Thermo and pH-responsive poly-N-isopropylacrylamide (PNIPAM) polymer has gained interest due to microenvironment targeting potential toward cancer cells. Its exceptional potential of the phase transition at body temperature (37°C) makes it biologically relevant for drug delivery and biosensing. The optimum drug loading and particle size with controlled release at a specific site are essential for critical process parameters (CPP). This study investigates the formulation optimization anastrozole (ANST)-loaded PNIPAM nanoparticle (NPs) prepared by solvent evaporation method for pH- and thermo-responsive drug delivery. Box-Behnken design (BBD) was implemented to observe the effect of selected process parameters on quality attributes product including particle size 110.15 nm, zeta potential − 11.02 mV, PDI 0.175, and drug loading 8.35% (DL). The statistical data was found to fit in the quadratic model and p value is less than 0.005. The thermo-responsive behavior of PNIPAM is evaluated on DLS and UV-Visible spectroscopy at elevated temperature to 60°C that has shown increment in turbidity showed aggregation of the nanoparticles. The TEM and AFM revealed the spherical and smooth surface ANST-PNIPAM NPs. The formulation showed the controlled release of ANST for 48 h at pH 7.4 and triggered release at simulated tumor microenvironment pH 5.0. The in vitro cytotoxicity of the formulation is higher than free ANST and shown dose-dependent cell viability. The higher cell uptake was observed by NPs after 12-h incubation in MCF-7 cell lines using confocal microscopy. Apoptotic evaluation of ANST-PNIPAM NPs exhibited 22.67% in comparison to free ANST where 6% was analyzed on the flow cytometer.













Similar content being viewed by others
References
Girgis A, Lambert S, Johnson C, Waller A, Currow D. Physical, psychosocial, relationship, and economic burden of caring for people with cancer: a review. J Oncol Pract. 2013;9(4):197–202.
Rivenbark AG, SM O€™C, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol. 2013;183(4):1113–24.
Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23(29):7350–60.
Olsen O, Gøtzsche PC. Cochrane review on screening for breast cancer with mammography. Lancet. 2001;358(9290):1340–2.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91.
Alyafee YA, Alaamery M, Bawazeer S, Almutairi MS, Alghamdi B, Alomran N, et al. Preparation of anastrozole loaded PEG-PLA nanoparticles: evaluation of apoptotic response of breast cancer cell lines. Int J Nanomedicine. 2018;13:199.
Sarkar K, Yang H. Encapsulation and extended release of anti-cancer anastrozole by stealth nanoparticles. Drug Delivery. 2008;15(5):343–6.
Shavi GV, Nayak UY, Maliyakkal N, Deshpande PB, Raghavendra R, Kumar AR, et al. Nanomedicine of anastrozole for breast cancer: physicochemical evaluation, in vitro cytotoxicity on BT-549 and MCF-7 cell lines and preclinical study on rat model. Life Sci. 2015;141:143–55.
Shukla R, et al. Microparticles of diethylcarbamazine citrate for the treatment of lymphatic filariasis. Asian J Chem. 2015;5(85):69047–56.
Pardhi VP, Verma T, Flora SJS, Chandasana H, Shukla R. Nanocrystals: an overview of fabrication, characterization and therapeutic applications in drug delivery. Curr Pharm Des. 2018;24:5129–46.
Gorain B, Choudhury H, Pandey M, Nair AB, Amin MCIM, Molugulu N, et al. Dendrimer-based nanocarriers in lung Cancer therapy. Nanotechnology-based targeted drug delivery systems for lung cancer: Elsevier. p. 161–92.
Jones ST, Walsh-Korb Z, Barrow SJ, Henderson SL, Js d B, Scherman OA. The importance of excess poly (N-isopropylacrylamide) for the aggregation of poly (N-isopropylacrylamide)-coated gold nanoparticles. ACS Nano. 2016;10(3):3158–65.
Pétriat F, Giasson S. Study of pH-sensitive copolymer/phospholipid complexes using the Langmuir balance technique: effect of anchoring sequence and copolymer molecular weight. Langmuir. 2005;21(16):7326–34.
Wu T, Ge Z, Liu S. Fabrication of thermoresponsive cross-linked poly (N-isopropylacrylamide) nanocapsules and silver nanoparticle-embedded hybrid capsules with controlled shell thickness. Chem Mater. 2011;23(9):2370–80.
Shaikh MV, Kala M, Nivsarkar M. Formulation and optimization of doxorubicin loaded polymeric nanoparticles using Box-Behnken design: ex-vivo stability and in-vitro activity. Eur J Pharm Sci. 2017;100:262–72.
Yang R, Nam K, Kim SW, Turkson J, Zou Y, Zuo YY, et al. Factorial design based multivariate modeling and optimization of tunable bioresponsive arginine grafted poly(cystaminebis(acrylamide)-diaminohexane) polymeric matrix based nanocarriers. Mol Pharm. 2017;14(2):252–63.
Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Nanomedicine. 6:683.
Garg NK, Sharma G, Singh B, Nirbhavane P, Tyagi RK, Shukla R, et al. Quality by design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): an improved dermatokinetic profile for inflammatory disorder(s). Int J Pharm. 2017;517(1–2):413–31.
Gaonkar RH, Ganguly S, Dewanjee S, Sinha S, Gupta A, Ganguly S, et al. Garcinol loaded vitamin E TPGS emulsified PLGA nanoparticles: preparation, physicochemical characterization, in vitro and in vivo studies. Sci Rep. 2017;7(1):530.
Mu L, Feng S-S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol®). J Control Release. 2002;80(1–3):129–44.
Li P-Y, Lai P-S, Hung W-C, Syu W-J. Poly(L-lactide)-vitamin E TPGS nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant MCF-7 breast cancer cells. Biomacromolecules. 2010;11(10):2576–82.
Hoo CM, Starostin N, West P, Mecartney ML. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J Nanopart Res. 2008;10(1):89–96.
Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008;101(2):239–53.
Bhattacharjee S. DLS and zeta potential: what they are and what they are not? J Control Release. 2016;235:337–51.
Zidan AS, Sammour OA, Hammad MA, Megrab NA, Hussain MD, Khan MA, et al. Formulation of anastrozole microparticles as biodegradable anticancer drug carriers. AAPS PharmSciTech. 2006;7(3):E38–46.
Bunjes H, Unruh T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv Drug Deliv Rev. 2007;59(6):379–402.
Ormategui N, Zhang S, Loinaz I, Brydson R, Nelson A, Vakurov A. Interaction of poly (N-isopropylacrylamide) (pNIPAM) based nanoparticles and their linear polymer precursor with phospholipid membrane models. Bioelectrochemistry. 2012;87:211–9.
Ingham B. X-ray scattering characterisation of nanoparticles. Crystallogr Rev. 2015;21(4):229–303.
Sun Y-P, Li X-q, Cao J, Zhang W-X, Wang HP. Characterization of zero-valent iron nanoparticles. Adv Colloid Interf Sci. 2006;120(1–3):47–56.
Van der Pol E, Coumans FAW, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12(7):1182–92.
De Jonge N, Ross FM. Electron microscopy of specimens in liquid. Nat Nanotechnol. 2011;6(11):695.
Li J, Piehler LT, Qin D, Baker JR, Tomalia DA, Meier DJ. Visualization and characterization of poly (amidoamine) dendrimers by atomic force microscopy. Langmuir. 2000;16(13):5613–6.
Zheng Y, Wang L, Lu L, Wang Q, Benicewicz BC. pH and thermal dual-responsive nanoparticles for controlled drug delivery with high loading content. ACS Omega. 2017;2(7):3399–405.
Kim S, Philippot S, Fontanay S, et al. pH- and glutathione-responsive release of curcumin from mesoporous silica nanoparticles coated using tannic acid–Fe(III) complex. RSC Adv. 2015;5(56):90550–8.
Sharma S, Verma A, Singh J, Teja BV, Mittapelly N, Pandey G, et al. Vitamin B6 tethered endosomal pH responsive lipid nanoparticles for triggered intracellular release of doxorubicin. ACS Appl Mater Interfaces. 2016;8(44):30407–21.
Shieh M-J, Hsu C-Y, Huang L-Y, Chen H-Y, Huang F-H, Lai P-S. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J Control Release. 2011;152(3):418–25.
Akrami M, Khoobi M, Khalilvand-Sedagheh M, Haririan I, Bahador A, Faramarzi MA, et al. Evaluation of multilayer coated magnetic nanoparticles as biocompatible curcumin delivery platforms for breast cancer treatment. RSC Adv. 2015;5(107):88096–107.
Na K, Lee ES, Bae YH. Adriamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J Control Release. 2003;87(1–3):3–13.
Urandur S, Banala VT, Shukla RP, Mittapelly N, Pandey G, Kalleti N, et al. Anisamide-anchored lyotropic nano-liquid crystalline particles with AIE effect: a smart optical beacon for tumor imaging and therapy. ACS Appl Mater Interfaces. 2017;10(15):12960–74.
Nallathamby PD, Xu X-HN. Study of cytotoxic and therapeutic effects of stable and purified silver nanoparticles on tumor cells. Nanoscale. 2010;2(16):942–52.
Emily A, Kristen K, Christin M, Elizabeth I, Donald A, Saber M. Tannic acid coated gold nanorods demonstrate a distinctive form of endosomal uptake and unique distribution within cells. ACS Appl Mater Interfaces. 2013;21(30):331–60.
Funding
Financial support was from the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India and Nanomission, India (Grant SR/NM/NS-1123/2016). The NIPER-R communication number is NIPER-R/Communication/062.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Vaishagya, K., K. Verma, R. et al. Temperature/pH-Triggered PNIPAM-Based Smart Nanogel System Loaded With Anastrozole Delivery for Application in Cancer Chemotherapy. AAPS PharmSciTech 20, 213 (2019). https://doi.org/10.1208/s12249-019-1410-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1410-3