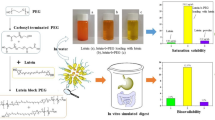
Abstract
Luteolin (LUT) and luteoloside (LUS) belong to flavonoids with high anticancer potential and were loaded into biodegradable diblock copolymer micelles of methoxy polyethylene glycol-polycaprolactone (mPEG5K-PCL10K), methoxy polyethylene glycol-polylactide-co-glycolide (mPEG5K-PLGA10K), and methoxy polyethylene glycol-polylactide (mPEG5K-PDLLA10K) by a self-assembly method, creating water-soluble LUT and LUS copolymer micelles, respectively. The solubilization formulations of the copolymer micelles were optimized with response surface methodology (RSM). The obtained drug micelles are torispherical under transmission electron microscope (TEM) with an average diameter of about 70 nm. The mPEG5K-PLGA10K exhibited higher loading capacity for LUS which was 4.33%, and LUT- (or LUS)-loaded mPEG5K-PCL10K exhibited a better stability and encapsulation efficiency which was 65.1 and 55.8%, respectively. The in vitro drug release study showed above 47% of LUT was released from micelles at pH 7.4 PBS; however, no more than 35% of LUT was released at pH 6.4 PBS within 24 h. Meanwhile, no more than 30% of LUS was released from micelles whether at pH 6.4 or 7.4 PBS solution within 24 h.




Similar content being viewed by others

REFERENCES
Pandurangan AK, Dharmalingam P, AnandaSadagopan SK, Ganapasam S. Effect of luteolin on the levels of glycoproteins during azoxymethane-induced colon carcinogenesis in mice. Asian Pac J Cancer Prev. 2012;13:1569–73.
Park SH, Park HS, Lee JH, Chi GY, Kim GY, Moon SK, et al. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem Toxicol. 2013;56:100–9.
Jeon Y, Suh YJ. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol Rep. 2013;29:819–25.
Zhang Q, Wan L, Guo Y, Cheng N, Cheng W, Sun Q, et al. Radiosensitization effect of luteolin on human gastric cancer SGC-7901 cells. J Biol Regul Homeost Agents. 2009;23:71–8.
Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem. 2004;265:107–13.
Veda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25:1197–202.
Liu R, Gao M, Qiang G, Zhang T, Lan X, Ying J, et al. The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience. 2009;162:1232–43.
Baskar AA, Ignacimuthu S, Michae GP, Numair KS. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr Cancer. 2011;63:130–8.
Tian Y, Sun L, Liu X, Li B, Wang Q, Dong J. Anti-HBV active flavone glucosides from Euphorbia humifusa Willd. Fitoterapia. 2010;81:799–802.
Rump AFE, Schqssler M, Acar D, Cordes A, Theisohn M, Rosen R, et al. Functional and antiischemic effects of luteolin-7-glucoside in isolated rabbit hearts. Gen Pharmacol. 1994;25:1137–42.
Shimoia K, Okadaa H, Furugoria M, Godaa T, Takasea S, Suzukib M, et al. Intestinal absorption of luteolin and luteolin-7-O-β-glucoside in rats and humans. FEBS Lett. 1998;438:220–4.
Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555:311–6.
Chen ZJ, Tu MJ, Sun SY, Kong SS, Wang YQ, Ye JF, et al. The exposure of luteolin is much lower than that of apigenin in oral administration of Flos Chrysanthemi extract to rats. Drug Metab Pharmacokinet. 2012;27:162–8.
Laskar P, Saha B, Ghosh SK, Dey J. PEG based random copolymer micelles as drug carriers: the effect of hydrophobe content on drug solubilization and cytotoxicity. RSC Adv. 2015;5:16265–76.
Nasongkla N, Shuai XT, Ai H, Weinberg BD, Pink J, Boothman DA, et al. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem. 2004;43:6323–7.
Gou ML, Men K, Shi HS, Xiang ML, Zhang J, Song J, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3:1558–67.
Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143:136–42.
Wang H, Zhao Y, Wu Y, Hu YL, Nan K, Nie G, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–90.
Majumdar D, Jung KH, Zhang HZ, Nannapaneni S, Wang X, Amin ARMR, et al. Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prev Res. 2014;7:65–73.
Qiu JF, Gao X, Wang BL, Wei XW, Gou ML, Men K, et al. Preparation and characterization of monomethoxy poly(ethylene glycol)-poly(ε-caprolactone) micelles for the solubilization and in vivo delivery of luteolin. Int J Nanomedicine. 2013;8:3061–9.
Zhou Q, Guo X, Chen T, Zhang Z, Shao SJ, Luo C, et al. Target-specific cellular uptake of folate-decorated biodegradable polymer micelles. J Phys Chem B. 2011;115:12662–70.
Zhao LY, Shi YK, Zou SH, Sun M, Li LB, Zhai GX. Formulation and in vitro evaluation of quercetin loaded polymeric micelles composed of pluronic P123 D-a-tocopheryl polyethylene glycol succinate. J Biomed Nanotechnol. 2011;7:358–65.
Wu W, Cui GH. Application of central composite design and response surface methodology in pharmacy. Foreign Med Sci Sect Pharm. 2000;27:292–8.
Murugesan K, Dhamija A, Nam IH, Kim YM, Chang YS. Decolourization of reactive black 5 by laccase: optimization by response surface methodology. Dyes Pigments. 2007;75:176–84.
Myers RH, Montgomery DC. In: Myers RH, editor. Response surface methodology: process and product in optimization using designed experiments. 2nd ed. New York: John Wily and Sons, Inc.; 2002.
Peng B, Zi J, Yan W. Measurement and correlation of solubilities of luteolinin organic solvents at different temperatures. J Chem Eng Data. 2006;51:2038–40.
Oh JK, Siegwart DJ, Lee H, Sherwood G, Peteanu L, Hollinger JO, et al. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J Am Chem Soc. 2007;129:5939–45.
Topel O, Cakır BA, Budama L, Hoda N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J Mol Liq. 2013;177:40–3.
Xu GY, Luan YX, Liu J, Yu L. Study on surfactant/macromolecule interaction by static fluorescence technology. Acta Phys -Chim Sin. 2005;21:577–82.
Allen C, Maysinger D, Eoisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B: Biointerfaces. 1999;16:3–27.
Lavasanifar A, Samuel J, Kwon GS. Poly (ethylene oxide)-block-poly(L-aminoacid) micelles for drug delivery. Adv Drug Deliv Rev. 2002;54:169–90.
Zeng X, Guo L, Ma LF. Preparation and characterization of glycyrrhetinic acid-modified poly(ethylene glycol)-poly(β-benzyl-L-asparate) nanoparticles as liver-targeted delivery system. Colloid Polym Sci. 2015;293:319–28.
Riley T, Govender T, Stolnik S, Xiong CD, Garnett MC, Lllum L, et al. Colloidal stability and drug incorporation aspects of micellar-like PLA-PEG nanoparticles. Colloids Surf B: Biointerfaces. 1999;16:147–59.
Mielczarek C. Acid–base properties of selected flavonoid glycosides. Eur J Pharm Sci. 2005;25:273–9.
Favaro G, Clementi A, Romani V. Acidichromism and ionochromism of luteolin and apigenin, the main components of the naturally occurring yellow weld: a spectrophotometric and fluorimetric study. J Fluoresc. 2007;17:707–14.
Amat A, Angelis FD, Sgamellotti A, Fantacci S. Acid-base chemistry of luteolin and its methy-ether derivatives: ADFT and ab initio investigation. Chem Phys Lett. 2008;462:313–7.
ACKNOWLEDGEMENTS
This work was supported by the Support Plan of Science and Technology Innovation team in Universities and Colleges in Henan Province of China (No. 14IRTSTHN030), Key Project of Science and Technology Research in Education Department of Henan Province in China (No. 14A150011), and Key Technology Research Program of Henan Province in China (No. 152102210257).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 309 kb)
Rights and permissions
About this article
Cite this article
Qing, W., Wang, Y., Li, H. et al. Preparation and Characterization of Copolymer Micelles for the Solubilization and In Vitro Release of Luteolin and Luteoloside. AAPS PharmSciTech 18, 2095–2101 (2017). https://doi.org/10.1208/s12249-016-0692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0692-y