ABSTRACT
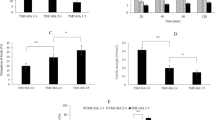
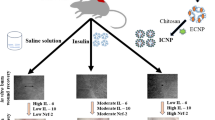
The aim of this study was to develop and characterize rh- IL-2 loaded chitosan-based nanogels for the healing of wound incision in rats. Nanogels were prepared using chitosan and bovine serum albumin (BSA) by ionic gelation method and high temperature application, respectively. Particle size, zeta potential, and polydispersity index were measured for characterization of nanogels. The morphology of nanogels was examined by using SEM and AFM. The IL-2 loading capacity of nanogels was determined using ELISA method. In vitro release of IL-2 from nanogels was performed using Franz diffusion cells. Artificial neural network (ANN) models were developed using selected input parameters (stirring rate, chitosan%, BSA%, TPP%) where particle size was an output parameter for IL-2 free nanogels. Wound healing effect of IL-2 loaded chitosan-TPP nanogel was evaluated by determining the malondialdehyde (MDA) and glutathione (GSH) levels of wound tissues in rats. The particle size of IL-2 loaded chitosan-TPP nanogels was found to be larger than that of IL-2 loaded BSA-based chitosan nanogels. Drug loading capacity of nanogels was found 100% ± 0.010 for both nanogels. IL-2 was released slowly after the initial burst effect. According to SEM and AFM imaging, BSA-chitosan nanogel particles were of nanometer size and presented a swelling tendency, and chitosan-TPP nanogel particles were found to be spherical and homogenously dispersed. IL-2 loaded chitosan-TPP nanogel was found suitable for improving wound healing because it decreased the MDA levels and increased the GSH levels wound tissues comparing to control group.





Similar content being viewed by others
REFERENCES
Vinogradov SV. Colloidal microgels in drug delivery applications. Curr Pharm Des. 2006;12:4703–12.
Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–49.
Kabanov AV, Vinogradov SV. Nanogels as pharmaceuticals carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418–29.
Yu S, Hu J, Pan X, Yao P, Jiang M. Stable and pH-sensitive nanogels by self-assembly of chitosan and ovalbumin. Langmuir. 2006;22:2754–9.
Lopez-Leon T, Carvalho ELS, Seijo B, Ortega-Vinuesa JL, Bartos-Gonzalez D. Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behaviour. J Colloid Interface Sci. 2005;283:344–51.
Debache K, Kropf C, Schütz CA, Harwood LJ, Kauper P, Monney T, et al. Vaccination of mice with chitosan nanogel-associated recombinant NcNDI against challenge infection with Neospora caninum tachyzoites. Parasite Immunol. 2011;33:81–94.
Schmitt F, Lagopoulos L, Käuper P, Rossi N, Busso N, Barge J, et al. Chitosan-based nanogels for selective delivery of photosensitizers to macrophages and improved retention in and therapy of articular joints. J Control Release. 2010;144:242–50.
Feng C, Guohui S, Zhiguo W, Xiaojie C, Hyunjin P, Dongsu C, et al. Transport mechanism of doxorubicin loaded chitosan based nanogels across intestinal epithelium. Eur J Pharm Biopharm. 2014;87:197–207.
Duan C, Zhang D, Wang F, Zheng D, Jia L, Feng F, et al. Chitosan-g-poly(N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int J Pharm. 2011;409:252–9.
Kim IS, Jeong YI, Kim SH. Self-assembled hydrogel nanoparticles composed of dextran and poly(ethylene glycol) macromer. Int J Pharm. 2000;205:109–16.
Carvalho V, Castanheira P, Madureira P, Ferreira SA, Conta C, Teixeira JP, et al. Self-assembled dextrin nanogel as protein carrier: controlled release and biological activity of IL-10. Biotechnol Bioeng. 2011;108:1977–86.
Gupta M, Gupta AK. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J Control Release. 2004;99:157–66.
Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, et al. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release. 1998;54:313–20.
Kobayashi H, Katakura O, Morimoto N, Akiyoshi K, Kasugai SJ. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. Biomed Mater Res B Appl Biomater. 2009;9:55–60.
Ferreira SA, Coutinho PJ, Gama FM. Synthesis and characterization of self-assembled nanogels made of pullulan. Materials. 2011;4:601–20.
Lee H, Mok H, Lee S, Oh YK, Park TG. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J Control Release. 2007;119:245–52.
Dickersen EB, Blackburn WH, Smith MH, Kapa LB, Lyon LA, McDonald JF. Chemosensitization of cancer cells by siRNA using targeted nanogel delivery. BioMed Cent Cancer. 2010. doi:10.1186/1471-2407-10-10.
Kim C, Lee Y, Lee SH, Kim JS, Jeong JH, Park TG. Self-crosslinked polyethylenimine nanogels for enhanced intracellular delivery of siRNA. Macromol Res. 2011;19:166–71.
Lemieux P, Vinogradov SV, Gebhart CL, Guérin N, Paradis G, Nguyen HK, et al. Block and graft copolymers and Nanogel™ copolymer networks for DNA delivery into cell. J Drug Target. 2000;8:91–105.
Vinogradov SV, Batrakova EV, Kabanov AV. Poly(ethylene glycol)–polyethylene imine NanoGel™ particles: novel drug delivery systems for antisense oligonucleotides. Colloids Surf B: Biointerfaces. 1999;16:291–304.
Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15:50–60.
Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367:330–5.
Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci U S A. 1996;93:10405–10.
Baiocchi RA, Caligiuri MA. Low dose interleukin 2 prevents the development Epstein-Barr virus (EBV) associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1994;91:5577–81.
Robb RJ, Kutny RM, Panico M, Morris HR, Chowdhry V. Amino acid sequence and post-translational modification of human interleukin 2. Proc Natl Acad Sci U S A. 1984;81:6486–90.
Pahwa R, Chatila T, Pahwa S, Paradise C, Day NK, Geha R, et al. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc Natl Acad Sci U S A. 1989;86:5069–73.
Artillo S, Pastore G, Alberti A, Miella M, Santantonio T, Fattovich G, et al. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J Med Virol. 1998;54:167–72.
Besien KV, Mehra R, Wadehra N, Stock W, Khouri I, Giralt S, et al. Phase II study of autologous transplantation with interleukin-2-incubated peripheral blood stem cells and posttransplantation interleukin-2 in relapsed or refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2004;10:386–94.
Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pract Oncol. 2007;4:497.
Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ. Interleukin 2 mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983;130:1784–9.
Dinarello CA. Biology of interleukin. FASEB J. 1988;2:108–15.
Barbul A, Knud-Jansen J, Wasserkrug HL, Efron G. Interleukin 2 enhances wound healing in rats. J Surg Res. 1986;4:315–9.
DeCunzo LP, Mackenzie JW, Marafino BJ, Devereux DF. The effect of interleukin-2 administration on wound healing in adriamycin-treated rats. J Surg Res. 1990;49:419–27.
Fleury L, Ollivon M, Dubois JL, Puisieux F, Barratt G. Preparation and characterization of dipalmitoylphosphatidylcholine liposomes containing interleukin-2. Braz J Med Biol Res. 1995;28:519–29.
Liu LS, Liu SQ, Ng SY, Froix M, Ohno T, Heller J. Controlled release of interleukin 2 for tumor immunotherapy using alginate/chitosan porous microspheres. J Control Release. 1997;43:65–74.
Cadeé JA, Groot CJ, Jiskoot W, Otter W, Hennink WE. Release of recombinant human interleukin-2 from dextran-based hydrogels. J Control Release. 2002;78:1–13.
Agatonovic-Kustrin S, Beresford R, Pauzi A, Yusof M. ANN modeling of the penetration across a polydimethylsiloxane membrane from theoretically derived molecular descriptors. J Pharm Biomed Anal. 2001;26:241–54.
Degim T, Hadgraft J, Ilbasmis S, Ozkan Y. Prediction of skin penetration using artificial neural network (ANN) modelling. J Pharm Sci. 2003;92:656–64.
Degim IT. Understanding skin penetration: computer aided modeling and data interpretation. Curr Comput Aided Drug Des. 2005;1:11–9.
Takayama K, Fujikawa M, Obata Y, Morishita M. Neural network based optimization of drug formulations. Adv Drug Deliv Rev. 2003;55:1217–31.
Celebi N, Erden N, Gönül B, Koz M. Effects of epidermal growth factor dosage forms on dermal wound strength. J Pharm Pharmacol. 1994;46:386–7.
Değim Z, Celebi N, Sayan H, Babul A, Erdoğan D, Take G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids. 2002;22:187–98.
Casini A, Ferrali M, Pompella A. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobebzene-intoxicated mice. Am J Pathol. 1986;123:520–31.
Özer Ç, Gönül B, Celebi N, Yetkin G, Elmas Ç, Erdoğan D. Effect of oral TGF-A formulations on ASA induced duodenal ulcer and the role of lipid peroxidation in the healing process. Turk J Biochem. 2012;37:294–302.
Chen Y, Mohanraj VJ, Wang F, Benson HA. Designing chitosan–dextran sulfate nanoparticles using charge ratios. AAPS Pharm Sci Tech. 2007;8:E98.
Peng H, Zhiming L, Hengyao H, Daxiang C. Synthesis and characterization of bovine serum albumin-conjugated copper sulfide nanocomposites. J Nanomater. 2010. doi:10.1155/2010/641545,6.
Yang L, Xie Z, Li Z. Studies on acrylate copolymer soap-free waterborne coatings crosslinked by metal ions. J Appl Polym Sci. 1999;74:91–6.
Rahimi M, Yousef M, Cheng Y, Meletis EI, Eberhart RC, Nguyen K. Formulation and characterization of a covalently coated magnetic nanogel. J Nanosci Nanotechnol. 2009;9:4128–34.
Shu XZ, Zhu KJ. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int J Pharm. 2000;201:51–8.
Oh NM, Oh KT, Baik HJ, Lee BR, Lee AH, Youn YS, et al. A self-organized 3-diethylaminopropyl-bearing glycol chitosan nanogel for tumor acidic pH targeting: in vitro evaluation. Colloids Surf B: Biointerfaces. 2010;78:120–6.
Hu J, Yu S, Yao P. Stable amphoteric nanogels made of ovalbumin and ovotransferrin via self-assembly. Langmuir. 2007;23:6358–64.
Nasti A, Zaki NM, Leonardis P, Ungphaiboon S, Sansongsak P, Rimoli MG, et al. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: systemic optimisation of the preparative process and preliminary biological evaluation. Pharm Res. 2009;26:1918–30.
Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier-systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B: Biointerfaces. 2007;59:24–34.
Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63.
Kılıç C, Güleç Peker EG, Acartürk F, Kılıçaslan SM, Çoşkun Cevher Ş. Investigation of the effects of local glutathione and chitosan administration on incisional oral mucosal wound healing in rabbits. Colloids Surf B: Biointerfaces. 2013;112:499–507.
Alemdaroğlu C, Değim Z, Celebi N, Zor F, Öztürk S, Erdoğan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319–27.
Bartone FF, Adickes ED. Chitosan effects on wound healing in urogenital tissue: preliminary report. Urology. 1988;140:1134–7.
Acknowledgements
This study was supported by Gazi University Scientific Research Foundation (Project Number: 02/2009-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no declaration of interest.
Additional information
Guest Editors: Claudio Salomon, Francisco Goycoolea, and Bruno Moerschbacher
Rights and permissions
About this article
Cite this article
Aslan, C., Çelebi, N., Değim, İ.T. et al. Development of Interleukin-2 Loaded Chitosan-Based Nanogels Using Artificial Neural Networks and Investigating the Effects on Wound Healing in Rats. AAPS PharmSciTech 18, 1019–1030 (2017). https://doi.org/10.1208/s12249-016-0662-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0662-4