Abstract
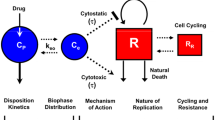
The time course of chemotherapeutic effect is often delayed relative to the time course of chemotherapeutic exposure. In many cases, this delay is difficult to characterize mathematically through the use of standard pharmacodynamic models. In the present work, we investigated the relationship between methotrexate (MTX) exposure and the time course of MTX effects on tumor cell growth in culture. Two cancer cell lines, Ehrlich ascites cells and sarcoma 180 cells, were exposed for 24 hours to MTX concentrations that varied more than 700-fold (0.19–140 μg/mL). Viable cells were counted on days 1, 3, 5, 7, 9, 11, 13, 15, 17, 20, 22, and 24 for Ehrlich ascites cells and on days 1, 2, 3, 5, 7, 9, 11, 13, 14, 15, 17, 19, and 21 for sarcoma 180 cells, through the use of a tetrazolium assay. Although MTX was removed 24 hours after application, cell numbers reached nadir values more than 100 hours after MTX exposure. Data from each cell line were fitted to 3 pharmacodynamic models of chemotherapeutic cell killing: a cell cycle phase-specific model, a phase-nonspecific model, and a transit compartment model (based on the general model recently reported by Mager and Jusko, Clin Pharmacol Ther. 70:210–216, 2001). The transit compartment model captured the data much more accurately than the standard pharmacodynamic models, with correlation coefficients ranging from 0.86 to 0.999. This report shows the successful application of a transit compartment model for characterization of the complex time course of chemotherapeutic effects; such models may be very useful in the development of optimization strategies for cancer chemotherapy.
Similar content being viewed by others
References
Lokich J, Anderson N. Dose intensity for bolus versus infusion chemotherapy administration: review of the literature for 27 anti-neoplastic agents. Ann Oncol. 1997;8:15–25.
Aisner J, Van Echo DA, Whitacre M, Wiernik PH. A phase I trial of continuous infusion VP16-213 (etoposide). Cancer Chemother Pharmacol. 1982;7:157–160.
Frei ED, Bickers JN, Hewlett JS, et al. Dose schedule and antitumor studies of arabinosyl cytosine (NSC 63878). Cancer Res. 1969;29:1325–1332.
Wiernik PH, Schwartz EL, Strauman JJ, et al. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–2493.
O Dwyer PJ, Hudes GR, Walczak J, et al. Phase I and pharmacokinetic study of the novel platinum analogue CI-973 on a 5-daily dose schedule. Cancer Res. 1992;52:6746–6753.
Minami H, Sasaki Y, Saijo N, et al. Indirect-response model for the time course of leukopenia with anticancer drugs. Clin Pharmacol Ther. 1998;64:511–521.
Minami H, Sasaki Y, Watanabe T, Ogawa M. Pharmacodynamic modeling of the entire time course of leukopenia after a 3-hour infusion of paclitaxel. Jpn J Cancer Res. 2001;92:231–238.
Friberg LE, Freijs A, Sandstrom M, Karlsson MO. Semiphysiological model for the time course of leukocytes after varying schedules of 5-fluorouracil in rats. J Pharmacol Exp Ther. 2000;295:734–740.
Jodrell DI, Egorin MJ, Canetta RM, et al. Relationships between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol. 1992;10:520–528.
Stewart CF, Baker SD, Heideman RL, et al. Clinical pharmacodynamics of continuous infusion topotecan in children: systemic exposure predicts hematologic toxicity. J Clin Oncol. 1994;12:1946–1954.
Cellarier E, Terret C, Labarre P, et al. Pharmacokinetic study of cystemustine, administered on a weekly schedule in cancer patients. Ann Oncol. 2002;13:760–769.
Van Kesteren C, Mathot RA, Raymond E, et al. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of the novel anticancer agent E7070 in four phase I studies. Br J Clin Pharmacol. 2002;53:553P.
Zhou H, Choi L, Lau H, et al. Population pharmacokinetics/toxicodynamics (PK/TD) relationship of SAM486A in phase I studies in patients with advanced cancers. J Clin Pharmacol. 2000;40:275–283.
Gimmel S, Maurer HR. Growth kinetics of L1210 leukemic cells exposed to different concentration courses of methotrexate in vitro. Cancer Chemother Pharmacol. 1994;34:351–355.
Braakhuis BJ, Ruiz van Haperen VW, Boven E, et al. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22:42–46.
Kishi S, Goto N, Nakamura T, Ueda T. Evaluation of cell-killing effects of 1-beta-D-arabinofuranosylcytosine and daunorubicin by a new computer-controlled in vitro pharmacokinetic simulation system. Cancer Res. 1999;59:2629–2634.
Mager DE, Jusko WJ. Pharmacodynamic modeling of time-dependent transduction systems. Clin Pharmacol Ther. 2001;70:210–216.
Jusko WJ. Pharmacodynamics of chemotherapeutic effects: dose-time-response relationships for phase-nonspecific agents. J Pharm Sci. 1971;60:892–895.
Jusko WJ. A pharmacodynamic model for cell-cycle-specific chemotherapeutic agents. J Pharmacokin Biopharm. 1973;1:175–200.
Sun YN, Jusko WJ. Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci. 1998;87:732–737.
Tada H, Shiho O, Kuroshima K, et al. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157–165.
D Argenio DZ, Schumitzky A. ADAPT II Users Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA: Biomedical Simulations Resource; 1997.
Levasseur LM, Slocum HK, Rustum YM, Greco WR. Modeling of the time-dependency of in vitro drug cytotoxicity and resistance. Cancer Res. 1998;58:5749–5761.
Hassan SB, Jonsson E, Larsson R, Karlsson MO. Model for time dependency of cytotoxic effect of CHS 828 in vitro suggests two different mechanisms of action. J Pharmacol Exp Ther. 2001;299:1140–1147.
Rodman JH, Relling MV, Stewart CF, et al. Clinical pharmacokinetics and pharmacodynamics of anticancer drugs in children. Semin Oncol. 1993;20:18–29.
Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505.
Sheiner LB, Stanski DR, Vozeh S, et al. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371.
Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–478.
Labat C, Mansour K, Malmary MF, et al. Chronotoxicity of methotrexate in mice after intraperitoneal administration. Chronobiologia. 1987;14:267–275.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published October 29, 2002
Rights and permissions
About this article
Cite this article
Lobo, E.D., Balthasar, J.P. Pharmacodynamic modeling of chemotherapeutic effects: Application of a transit compartment model to characterize methotrexate effects in vitro. AAPS J 4, 42 (2002). https://doi.org/10.1208/ps040442
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps040442