Abstract
For nearly 100 y, pediatricians have regularly used oxygen to treat neonatal and childhood diseases. During this time, it has become clear that oxygen is toxic and that overzealous use can lead to significant morbidity. As we have learned more about the appropriate clinical indications for oxygen therapy, studies at the bench have begun to elucidate the molecular mechanisms by which cells respond to hyperoxia. In this review, we discuss transcription factors whose activity is regulated by oxygen, including nuclear factor, erythroid 2-related factor 2 (Nrf2), activator protein 1 (AP-1), p53, nuclear factor κB (NF-κB), signal transducers and activators of transcription protein (STAT), and ccat/enhancer binding protein (CEBP). Special attention is paid to the mechanisms by which hyperoxia affects these transcription factors in the lung. Finally, we identify downstream targets of these transcription factors, with a focus on heme oxygenase-1. A better understanding of how oxygen affects various signaling pathways could lead to interventions aimed at preventing hyperoxic injury.
Similar content being viewed by others
Main
Oxygen therapy has a long and tortuous history in Neonatology. The pendulum has swung from a liberal use of supplemental oxygen in the early 20th century, to limited application in the 1950s based on the association with retinopathy of prematurity. Today, clinical studies are focused on addressing which neonatal pathologic states require treatment with oxygen, and what level of oxygen administration is safe. In concert with these clinical studies, much work has been done at the bench to ascertain how oxygen affects gene expression. This is of particular relevance in neonates because changes in gene expression at critical times in development can have long-lasting effects and subsequent consequences on lung structure and function. This review will address lessons learned and new insights as to the effects of hyperoxia on pulmonary gene expression.
EVOLUTIONARY PERSPECTIVE
Responses to atmospheric oxygen have evolved in eukaryotes during the last 1.5 billion years (1). The ability of organisms to reduce oxygen to water critically altered cellular metabolism and energy production, but also resulted in the formation of toxic reactive oxygen species (ROS) via the mitochondrial respiratory chain. These radicals are electron donors, which can damage DNA, RNA, protein, and lipids. They can also propagate deleterious reactions throughout cells and tissues resulting in death and apoptosis. In addition, these ROS can alter gene expression by modulating transcription factor activation, which then impact downstream targets. In oxygen breathing animals, only three tissues—the cornea, the skin, and the respiratory tract epithelium—are exposed to 21% oxygen, equivalent to a partial pressure of about 160 mm Hg at sea level. The remaining tissues are exposed to much lower oxygen tensions. The affinity of Hb for oxygen maintains the Po2 in the mitochondria below 0.5 mm Hg, limiting the production of ROS and effectively protecting the body from oxygen toxicity (2). Before the advent of the medical use of oxygen, humans were rarely exposed to oxygen tensions that were greater than those in their ambient environment. Thus, it stands to reason that evolution may not have dictated a well-developed response to acute increases in oxygen tension. The notable exception is the transition at birth from the womb to the outside world where we were rapidly shifted from a relative hypoxic environment to relative hyperoxia. Additionally, the lung epithelium is constantly exposed to “relative hyperoxia” compared with other tissues and is further stressed by oxygen therapy.
HISTORICAL PERSPECTIVE
From the time of its discovery in the 1770s, oxygen has held promise as an elixir for multiple human ailments. Within 10 y of its discovery, Anton Lavoisier applied oxygen to newborn infants requiring resuscitation (3). By the early 1900s, physicians were administering oxygen to treat cyanosis in premature infants (4). Shortly thereafter, oxygen therapy became widespread in neonatal units, with therapeutic indications ranging from respiratory distress to periodic breathing. However, by the early 1950s, published reports linking oxygen to the pathogenesis of retinopathy of prematurity began to appear, and the use of oxygen was quickly curtailed (5). Nevertheless, physicians were reminded that oxygen was a powerful and life-saving therapy when increased mortality from hyaline membrane disease (6) and the resurgence of cerebral palsy (7) were observed. This demonstrated that both too much and too little oxygen were problematic. Vigorous debates about the appropriate use of oxygen during newborn resuscitation (8) and the proper pulse oximetry saturation goals for premature infants (9) currently rage on. At this time, six multicenter randomized controlled trials are attempting to define optimal oxygen therapy goals for preterm babies (9).
Studies at the bench pair nicely with these clinical trials. Investigators have used multiple in vivo and in vitro models to determine how oxygen affects gene expression and subsequent lung structure and function. Hyperoxia results in alveolar and endothelial cell destruction, fluid leak into the air space, respiratory failure, and mortality (10). The lungs of animals exposed to hyperoxia show increased mean linear intercepts, influx of macrophages, extracellular matrix turnover, and fibrin deposition (11). During hyperoxia, ROS are produced both by the electron transport chain in the mitochondria and by the membrane-bound NADPH oxidase (12–15). ROS cause DNA strand breaks and other chromosomal aberrations (16,17), which stimulate the expression of genes involved in inhibiting cell cycle progression (18). There is clear evidence in animal models that exposure to hyperoxia results in lung morphology similar to that of bronchopulmonary dysplasia (BPD) (11,19). These studies serve as important correlates to the ongoing trials involving oxygen therapy for premature infants.
HYPEROXIC GENE REGULATION
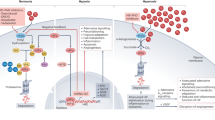
Organs, tissues, and cells have evolved systems to rapidly respond to changes in their microenvironment. A stimulus, which causes a perturbation, must be detected and translated into a response, which then facilitates a return to the steady state (Fig. 1). Receptors, signaling pathways, transcription factors, and downstream changes in proteins and metabolic function have evolved for this purpose. Only a few transcription factors that specifically alter gene expression in response to increased oxygen tension have been identified, as well as some direct downstream targets (Table 1). These will be discussed later.
How a stimulus is perceived and how cells respond to return to the steady state. Cellular receptors or sensors detect stimuli such as hyperoxia. This leads to the translation of this signal via signal transduction pathways, which result in transcription factor activation. This then generates a response such as gene regulation and subsequent protein synthesis and a return to the steady state.
TRANSCRIPTION FACTORS RESPONSIVE TO HYPEROXIA
Nrf2.
The detoxification of ROS and electrophiles is important to prevent cellular injury (Fig. 2). The transcription factor nuclear factor, erythroid 2-related factor 2 (Nrf2) regulates the inducible expression of a group of detoxification enzymes, such as glutathione S-transferase and NAD(P)H:quinone oxidoreductase, via antioxidant response elements (ARE). Under normal circumstances, Nrf2 is retained in the cytoplasm by a repressor protein Kelch-like ECH-associated protein 1 (Keap1). Exposure to xenobiotics and oxidants leads to the dissociation of Nrf2 from Keap1, which allows the free Nrf2 to translocate to the nucleus where it heterodimerizes with c-Jun, an activator protein 1 (AP-1) family protein (20). The consensus binding sequence of Nrf2 shows high similarity to the ARE/electrophile-responsive element sequence previously identified (21–23). Nrf2 can also heterodimerize with small Maf proteins to regulate ARE-mediated gene expression (24). These Maf proteins are so named because of their structural similarity to the founding member, the oncoprotein v-Maf. They include a characteristic basic region linked to a leucine zipper (b-Zip) domain, which mediate DNA binding and subunit dimerization, respectively (25).
Lung Nrf2 responds to hyperoxia (26). Linkage analysis identified Nrf2 as an important mediator of protection against lung hyperoxic injury (27) and mice deficient in Nrf2 exhibit aggravated lung injury and a lack of upregulation of ARE-mediated phase 2 detoxifying and antioxidant enzymes (28). Further gene array analysis of wild type vs. Nrf2-deficient mice revealed discordance in multiple genes, thus identifying potential downstream targets of this important transcription factor (29). In fact, a single nucleotide polymorphism found in the Nrf2 promoter increases the risk of acute lung injury in human subjects (30). This evidence provides an important translational correlate and may lead to the development of therapeutic strategies.
AP-1.
AP-1 was first identified as a transcriptional factor that binds to an essential cis-element of the human metallothionein II gene (31). It is composed of fos and jun protein dimers that bind via hydrophobic interactions of their leucine-zipper regions (32). The jun/jun and jun/fos dimers form the AP-1 complex. This transcription factor controls genes involved in cellular proliferation and death in response to various stimuli including hyperoxia. The consensus AP-1-binding site is embedded in the ARE where fos and jun proteins may heterodimerize to Nrf2 in the presence of electrophiles and oxidants as discussed earlier (33). Blocking AP-1 activation enhances hyperoxia-induced cell death in murine lung epithelial cells (34,35). One specific target of hyperoxia-induced JNK1/AP-1 activation in A549 cells is the IL-8 promoter (36). This could modulate inflammatory responses with hyperoxic exposure. It is interesting to note that neonatal mice exposed to hyperoxia show no increase in lung AP-1 consensus sequence binding (37) in contrast to their adult counterparts (37,38). However, in the brain, increased AP-1 consensus sequence binding occurs in the forebrain and hippocampus of both adult and younger rats exposed to hyperoxia (39,40). These data suggest both maturational differences and tissue specificity of AP-1 activation.
p53.
The transcription factor p53 regulates the expression of a large number of target genes including those related to cell cycle arrest, cell death, and DNA repair (41). Since its discovery in 1979, p53 has been identified as a tumor suppressor and its role in human cancer has become clearer (42). Under basal conditions, p53 resides in the cytoplasm and is subjected to ubiquitin-mediated proteolysis. However, in response to stimuli such as DNA damage, p53 is phosphorylated, stabilized, and enters the nucleus (41). Under conditions of cellular stress, activated p53 initiates growth arrest and induces proapoptotic gene expression (42). Hyperoxia increases p53 gene transcription, protein levels, and activity (16,43–45). In preterm baboons, exposure to hyperoxia results in increased p53 protein leted p53 initiates growth arrest and induces proapoptotic gene expression (42). Hyperoxia increases p53 gene transcription, protein levels, and activity (16,43–45). In preterm baboons, exposure to hyperoxia results in increased p53 protein levels in airway epithelium (46,47). However, in p53−/− mice exposed to hyperoxia, lung injury and lethality did not differ from similarly exposed wild-type animals (16,48). These data indicate that the exact role of p53 in modulating the cellular response to hyperoxia remains to be elucidated.
NF-κB.
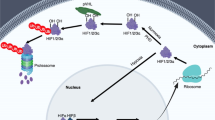
The nuclear factor kappa B (NF-κB) family is composed of highly conserved dimeric proteins, which activate genes that regulate apoptosis, inflammation, and oxidative stress (49–51) (Fig. 3). This factor regulates gene expression and was first described by Baltimore and Sen (52). In quiescent cells, NF-κB dimers remain sequestered in the cytoplasm bound to a member of the κB (IκB) family of inhibitory proteins (50). IκBα is the prototypical member of this family and the most well studied. With inflammatory or oxidant stress, IκBα is phosphorylated, resulting in dissociation and unmasking of the nuclear localization sequence of NF-κB (51). After inflammatory stimuli, such as TNF-α activation, IκBα is phosphorylated on serine 32/36 and degraded through the proteosomal pathway (51). In addition to this canonical pathway, an atypical pathway of NF-κB activation results from specific phosphorylation of IκBα on tyrosine 42 (53). This occurs after stimulation with pervanadate, nerve growth factor (NGF), hydrogen peroxide, and ischemia-reperfusion (53–55) and, as most recently demonstrated, with hyperoxia (56). This latter pathway represents an intriguing molecular target for modulating the pulmonary response to hyperoxia.
NF-κB-mediated gene expression. With hyperoxia, there is phosphorylation (p) of the inhibitory protein IκBα on tyrosine 42. This results in the ubiquination (u) and subsequent degradation of IκBα. This allows for dissociation and nuclear translocation of the active NF-κB complex (p65 and p50 are represented here), binding to consensus sequences on various genes and transcriptional activation or repression of gene expression.
It is important to note that NF-κB nuclear translocation and DNA binding can either enhance or suppress target gene expression. The subunit composition of the NF-κB dimer likely confers specificity to the expression of target genes after activation (57). The most abundant NF-κB protein is the p65-p50 dimer (58). The p65 subunit contains a transactivation domain that interacts with other transcription proteins to increase gene expression (59). The p50 subunit lacks this transactivation domain, and can repress transcription when bound to DNA as a p50-p50 homodimer (59,60). Furthermore, the ability of NF-κB to alter gene expression is affected by posttranslational modifications including phosphorylation and acetylation (59).
Hyperoxia-induced NF-κB activation appears to be stimulus and cell type specific. Nuclear translocation of NF-κB was shown in A549 lung adenocarcinoma cells exposed to hyperoxia-induced but this activation did not protect against cell death (61). Also, in adult mice exposed to hyperoxia, NF-κB activated proinflammatory markers in pulmonary lymphocytes (62). Furthermore, in fetal mouse lung explants, hyperoxia-induced NF-κB activation was associated with increased apoptosis which was reversed by blocking NF-κB activation (63). In contrast, inhibition of hyperoxia-induced NF-κB activation accelerated nonapoptotic cell death in primary and transformed lung epithelial cells, resulting in decreased levels of MnSOD (64). Additionally, A549 cells pretreated with hyperoxia showed less apoptosis after exposure to hydrogen peroxide, an effect reversed by inhibiting NF-κB activation (65). In other examples, NF-κB was not activated in response to hyperoxic exposure (66,67), suggesting that this signaling pathway is cell specific. The lung contains over 40 different cell types (68), and the response to hyperoxia is cell type specific. Endothelial cells are very sensitive to oxygen toxicity, whereas type II epithelial cells are resistant and proliferate in the recovery phase (69). Furthermore, in the developing lung, exposure to hyperoxia prevents the normal differentiation of type II cells to type I cells in the developing lung (70). Further studies are necessary to fully dissect the specificity and complexity of hyperoxia-induced NF-κB activation. Nevertheless, these findings suggest that interventions to either inhibit or enhance NF-κB activation in hyperoxia could be of therapeutic benefit.
Various clinical interventions, such as glucocorticoids, can inhibit NF-κB activation (71–74). Adrenalectomized adult mice exposed to hyperoxia had less lung injury and had improved survival due to increased NF-κB activation (75). Thus, hyperoxia-induced NF-κB activation, when not limited by endogenous glucocorticoids, protects the adult lung from oxygen toxicity (71). Interestingly, after glucocorticoid therapy for BPD, cells obtained from tracheobronchial lavage fluid of premature neonates showed inhibition of NF-κB activation (76). Nitric oxide, which may prevent BPD in some infants (77), also inhibits NF-κB activity (78). The clinical implications of these findings remain to be explored in humans.
Of particular interest to pediatricians are the maturational differences found in NF-κB activation. Multiple models have shown increased NF-κB activation in neonates compared with adults after exposure to inflammatory and oxidant stimuli (79–81). In rat fetal alveolar type II cells, NF-κB translocates to the nucleus and binds DNA after hyperoxic exposure (82). This binding peaks soon after birth and gradually decreases postnatally, suggesting that NF-κB regulates genes involved in the transition from the relative hypoxic environment seen in utero (82). This activation may have important downstream effects as shown in hyperoxia exposed fetal lung fibroblasts where NF-κB activation prevented apoptosis through the suppression of proapoptotic genes (56). In contrast, this hyperoxic activation of NF-κB was not seen in adult lung fibroblasts (57). In the only published study evaluating hyperoxia-induced NF-κB activation in a neonatal in vivo model, Yang et al. showed that hyperoxia-induced NF-κB occurred in the lungs of neonatal but not in adult mice (81). This activation was associated with the relative tolerance to hyperoxic injury in the neonatal animals when compared with adults, and this tolerance was reversed when hyperoxia-induced NF-κB activation was inhibited (81). In contrast, clinical studies show that enhanced NF-κB activation is linked to respiratory distress syndrome and an increased risk of developing BPD in preterm infants (83–85). Thus, it is not yet clear whether inhibition of lung NF-κB is beneficial or harmful in human neonates.
The hyperoxic activation of NF-κB has also been investigated in tissues other than the lung. Using a bioluminescent NF-κB reporter mouse line, Dohlen et al. showed increased NF-κB activity in the brain after resuscitation with 100% O2 (86). In other studies, hyperoxia without preceding ischemia decreased NF-κB activation in the basal forebrain, with a more pronounced effect in aged vs. young mice (87).
It is clear that the NF-κB–mediated response to oxygen is influenced by maturation. Whether these changes are beneficial or detrimental remain to be seen. Understanding the maturational differences in hyperoxia-induced NF-κB activation could help guide interventions aimed to modulate this response in neonates.
STAT.
Another important transcription factor involved in hyperoxic gene regulation is the signal transducers and activators of transcription protein (STAT). This family of proteins is activated by various cell surface receptors in response to ligands, including cytokines, growth factors, and peptides (88). Hyperoxic lung injury is attenuated in mice constitutively expressing Stat3 in respiratory epithelial cells (89). Conversely, mice with disruption of Stat3 in respiratory epithelial cells demonstrate exaggerated hyperoxic lung injury and increased expression of proinflammatory cytokines including IL-6 (90).
CEBP.
The ccat/enhancer binding protein (C/EBP) family of proteins are basic leucine zipper transcription factors that respond to extracellular signals to regulate cell proliferation, differentiation, and tissue development (91). C/EBPβ and C/EBPδ consensus sequence binding was increased in the lungs of young and aged mice exposed to hyperoxia (38). In the mouse exposed to hyperoxia, there is downregulation of the protective Clara cell secretory protein (CCSP) due to enhanced C/EBPβ nuclear translocation and binding to the CCSP promoter (92). These studies are particularly relevant because C/EBPα is required for lung maturation (93).
Other transcription factors regulated by hyperoxia.
Acute and chronic exposure to hyperoxia may result in activation of a variety of other transcription factors including cmyc, fos-related antigen (Fra)-1, junB, c-fos as well as NGF1-A and -B (94). Furthermore, in the neonatal lung, hyperoxia can cause downregulation of sox-7 and sox-18 (94). The relevance of these signaling events is not fully clear.
SPECIFIC DOWNSTREAM GENE TARGETS OF HYPEROXIA
Because transcription factors that are regulated in hyperoxia control a multitude of genes, it would be difficult to list all of these genes (Table 1). For example, the activation of NF-κB can regulate the expression of over 100 genes. Nevertheless, only a small fraction of NF-κB responsive genes are activated in hyperoxia. Some of the genes regulated by Nrf-2 and NF-κB will be highlighted below.
Nrf-2-regulated genes.
Nrf2 binds to the ARE, driving the expression of genes including antioxidants such as glutathione peroxidase, catalase, superoxide dismutase, thiol metabolism-associated detoxifying enzymes such as glutathione-s-transferase and stress-response genes such as heme oxygenase-1 (HO-1), among others (25–28). These genes are all highly responsive to hyperoxia. We will focus on HO-1 as an example of an Nrf-2 regulated gene regulated in hyperoxia.
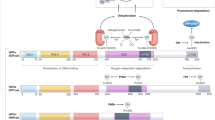
The HO-1 gene encodes for the rate-limiting enzyme in the degradation of heme and the formation of biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase. In recent years, many roles have been identified for this protein and it has been clearly demonstrated that HO-1 is a generalized response to oxidative stress (95). The mouse HO-1 gene is 6.8 kb in length and organized into four introns and five exons. A promoter sequence is located 28 base pairs (bps) upstream of the transcription initiation site. There is a proximal enhancer (PE) directly upstream of the promoter and there are two more distal enhancers located at 4 kb (DE1) and 10 kb (DE2) upstream of the transcription initiation site. Each enhancer region contains multiple transcription factor binding sites including composite AP-1 and NF-E2 or CREB/ATF sites (Fig. 4) (96–98). Induction of HO-1 in oxidative stress is via Nrf2 and small Maf proteins binding to the ARE (99). Competitive binding between Nrf2 and BTB and CNC homology 1, basic leucine zipper transcription factor 1 (Bach1), at the ARE is important in heme-mediated regulation of HO-1 (100). Several investigators have documented hyperoxic induction of HO-1 in adult mice. However, in the neonatal rodent HO-1 induction is limited. In the neonatal mouse and rat, hyperoxic exposure did not result in a significant increase in HO-1 mRNA as it did in similarly exposed adult (101,102). In another study, lung HO-1 mRNA only increased after 10 d of hyperoxic exposure in neonatal mice (94) whereas this occurred within 24 h in adult mice (103). There may be some teleological wisdom in not further elevating the levels of HO-1 when they are already quite high at birth and in the neonatal period, especially if this could lead to deleteriously high levels thus aggravating hyperoxic injury (104). We have also observed increased protein levels and DNA binding for Bach1, an inhibitor of HO-1 transcriptional activation, in neonates at baseline and after exposure to hyperoxia compared with adults (102). Typically, Bach1 is degraded in the presence of ROS (105). Enhanced Bach1 expression could ensure that there are sufficient levels for HO-1 gene inhibition in the neonate.
Diagram of the HO-1 gene. Numbers indicate base pairs. There are two DE. These contain a multiple antioxidant response element/stress response element (MARE/StRE), which has consensus sequence for a cadmium response element as well as an AP-1 binding site. The gene also contains a PE and a promoter (P).
NF-κB regulated genes.
The IGF-binding protein (IGFBP)2 promoter has NF-κB consensus sequence binding sites, and both NF-κB consensus sequence binding and IGFBP2-promoter reporter activity increase in response to hyperoxia (106). This binding protein inhibits DNA synthesis and cellular entry into the S-phase, indicating a role for hyperoxia-induced NF-κB activation in modulating oxygen toxicity in the lung. Methylprednisolone treatment inhibits hyperoxia-induced NF-κB activation and down-regulates ICAM-1 expression in human pulmonary artery endothelial cells (107), resulting in less neutrophil adhesion to the endothelium. As discussed earlier, adrenalectomized mice show attenuation of hyperoxic lung injury, and this is associated with preservation of NF-κB activation and induction of IL-6 (71). This cytokine is under the exclusive regulation of NF-κB with inflammation (108,109). Whether IL-6 is exclusively regulated by NF-κB in response to hyperoxia is not known. Nevertheless, IL-6 is enhanced in the lungs of neonatal and adult mice in response to hyperoxia (110), although this phenomenon is not consistently observed in adult mice (62,103). The amiloride-sensitive sodium channel, epithelium sodium channel (ENac), responsible for sodium and fluid absorption from the alveolar space (111), has an NF-κB binding site (112), and both NF-κB activation and ENaC gene expression increase with relative hyperoxia (113,114). Furthermore, hyperoxia-induced ENaC expression is prevented with NF-κB blockade (114) in some reports but not others (115,116).
Cell cycle genes.
Another important effect of hyperoxia is the modulation of genes involved in cell cycle regulation. Both acute and chronic exposures to hyperoxia result in upregulation of p21 (94). Of note, NF-κB is known to regulate the expression of p21 in some cells (117). This key inhibitor of cell cycle regulation and cellular proliferation is increased in both the neonatal (118) and adult (119) lung after exposure to hyperoxia. Expression of this protein in response to hyperoxia relies on either TGF-β signaling (120) or p53 activation (121,122). Upregulation of p21 is protective against hyperoxic injury in both neonatal (123) and adult (124) mice. It is hypothesized that inhibition of cellular proliferation during periods of oxidative stress allows for additional time to repair damaged DNA (125) thus providing cytoprotection.
CONCLUSION
Hyperoxia regulates multiple transcription factors in the lung. These, in turn, regulate a variety of downstream targets including ARE-regulated genes such as HO-1, antioxidant enzymes that are important in the detoxification of electrophiles, as well as genes involved in cell cycle regulation and the inflammatory response. The overall effect of hyperoxia in the lung depends on the maturational stage of the organism. The net effect of hyperoxic lung gene regulation may be both enhanced cytoprotection and worsened lung function. In the neonate where postnatal lung development is crucial to proper alveolar formation, hyperoxic gene regulation may have long-lasting effect on lung structure and function. A further understanding of how hyperoxia affects specific signaling pathways and subsequent gene expression could lead to interventions aimed at preventing hyperoxic injury.
Abbreviations
- AP-1:
-
activator protein 1
- ARE:
-
antioxidant response elements
- Bach1:
-
basic leucine zipper transcription factor 1
- BPD:
-
bronchopulmonary dysplasia
- C/EBP:
-
ccat/enhancer binding protein
- ENaC:
-
epithelium sodium channel
- HO-1:
-
heme oxygenase-1
- IκB:
-
inhibitor of κB
- Keap 1:
-
Kelch-like ECH-associated protein 1
- NF-κB:
-
nuclear factor κB
- Nrf2:
-
nuclear factor, erythroid 2 related factor 2
- ROS:
-
reactive oxygen species
- STAT:
-
signal transducers and activators of transcription protein
References
Raymond J, Segre D 2006 The effect of oxygen on biochemical networks and the evolution of complex life. Science 311: 1764–1767
Richmond S, Goldsmith JP 2006 Air or 100% oxygen in neonatal resuscitation?. Clin Perinatol 33: 11–27 vv
Silverman WA 2004 A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics 113: 394–396
Robertson AF 2003 Reflections on errors in neonatology: I. The “Hands-Off” years, 1920 to 1950. J Perinatol 23: 48–55
Silverman WA 1980 Retrolental Fibroplasia: A Modern Parable. Grune and Stratton, New York
Avery ME 1960 Recent increase in mortality from hyaline membrane disease. J Pediatr 57: 553–559
McDonald AD 1963 Cerebral palsy in children of very low birth weight. Arch Dis Child 38: 579–588
Saugstad OD, Ramji S, Soll RF, Vento M 2008 Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 94: 176–182
Tin W, Gupta S 2007 Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal Ed 92: F143–F147
Crapo JD 1986 Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 48: 721–731
Warner BB, Stuart LA, Papes RA, Wispé JR 1998 Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 275: L110–L117
Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM 2006 Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34: 453–463
Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V 2003 Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–L38
Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch A, Orosz CG, Sen CK 2003 Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res 92: 264–271
Chandel NS, Budinger GR 2007 The cellular basis for diverse responses to oxygen. Free Radic Biol Med 42: 165–174
Barazzone C, Horowitz S, Donati Y, Rodriguez I, Piguet PF 1998 Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 19: 573–581
O'Reilly MA 2001 DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 281: L291–L305
Roper JM, Gehen SC, Staversky RJ, Hollander MC, Fornace AJ Jr, O'Reilly MA 2005 Loss of Gadd45a does not modify the pulmonary response to oxidative stress. Am J Physiol Lung Cell Mol Physiol 288: L663–L671
Bonikos DS, Bensch KG, Ludwin SK, Northway WH 1975 Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest 32: 619–635
Jaiswal AK 2000 Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med 29: 254–262
Friling RS, Bensimon A, Tichauer Y, Daniel V 1990 Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA 87: 6258–6262
Rushmore TH, Morton MR, Pickett CB 1991 The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem 266: 11632–11639
Wasserman WW, Fahl WE 1997 Functional antioxidant responsive elements. Proc Natl Acad Sci USA 94: 5361–5366
Dhakshinamoorthy S, Jaiswal AK 2002 c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction. Oncogene 21: 5301–5312
Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD 1997 The world according to Maf. Nucleic Acids Res 25: 2953–2959
Cho HY, Reddy SP, Kleeberger SR 2006 Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87
Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR 2002 Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol 26: 42–51
Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR 2002 Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175–182
Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR 2005 Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med 38: 325–343
Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR 2007 Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 21: 2237–2246
Lee W, Haslinger A, Karin M, Tjian R 1987 Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature 325: 368–372
Angel P, Karin M 1991 The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157
Gong P, Stewart D, Hu B, Vinson C, Alam J 2002 Multiple basic-leucine zipper proteins regulate induction of the mouse heme oxygenase-1 gene by arsenite. Arch Biochem Biophys 405: 265–274
Romashko J, Horowitz S, Franek WR, Palaia TA, Miller EJ, Lin A, Birrer MJ, Scott W, Mantell L 2003 MAPK pathways mediate hyperoxia-induced oncotic cell death in lung epithelial cells. Free Radic Biol Med 35: 978–993
Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA 2003 Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 29: 779–783
Joseph A, Li Y, Koo HC, Davis JM, Pollack S, Kazzaz JA 2008 Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic Biol Med 45: 1143–1149
Yang G, Madan A, Dennery PA 2000 Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol 278: L393–L398
Choi AM, Sylvester S, Otterbein L, Holbrook NJ 1995 Molecular responses to hyperoxia in vivo: relationship to increased tolerance in aged rats. Am J Respir Cell Mol Biol 13: 74–82
Tong L, Toliver-Kinsky T, Rassin D, Werrbach-Perez K, Perez-Polo JR 2003 Hyperoxia increases AP-1 DNA binding in rat brain. Neurochem Res 28: 111–115
Tong L, Toliver-Kinsky T, Edwards M, Rassin DK, Werrbach-Perez K, Perez-Polo JR 2002 Attenuated transcriptional responses to oxidative stress in the aged rat brain. J Neurosci Res 70: 318–326
Horn HF, Vousden KH 2007 Coping with stress: multiple ways to activate p53. Oncogene 26: 1306–1316
Vogelstein B, Lane D, Levine AJ 2000 Surfing the p53 network. Nature 408: 307–310
O'Reilly MA, Staversky RJ, Stripp BR, Finkelstein JN 1998 Exposure to hyperoxia induces p53 expression in mouse lung epithelium. Am J Respir Cell Mol Biol 18: 43–50
Vaziri H, West MD, Allsopp RC, Davison TS, Wu YS, Arrowsmith CH, Poirier GG, Benchimol S 1997 ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the posttranslational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J 16: 6018–6033
Shenberger JS, Dixon PS 1999 Oxygen induces S-phase growth arrest and increases p53 and p21(WAF1/CIP1) expression in human bronchial smooth-muscle cells. Am J Respir Cell Mol Biol 21: 395–402
Das KC, Ravi D, Holland W 2004 Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal 6: 109–116
Maniscalco WM, Watkins RH, Roper JM, Staversky R, O'Reilly MA 2005 Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res 58: 549–556
O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC 2000 p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 278: L552–L559
Baeuerle PA, Baltimore D 1996 NF-kappa B: ten years after. Cell 87: 13–20
Hoffmann A, Baltimore D 2006 Circuitry of nuclear factor kappa B signaling. Immunol Rev 210: 171–186
Janssen-Heininger YM, Poynter ME, Baeuerle PA 2000 Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappa B. Free Radic Biol Med 28: 1317–1327
Sen R, Baltimore D 1986 Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47: 921–928
Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF 1996 Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell 86: 787–798
Koong AC, Chen EY, Giaccia AJ 1994 Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res 54: 1425–1430
Bui NT, Livolsi A, Peyron JF, Prehn JH 2001 Activation of nuclear factor kappa B and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of I kappa B alpha. J Cell Biol 152: 753–764
Wright CJ, Zhuang T, La P, Yang G, Dennery PA 2009 Hyperoxia-induced NF-{kappa}B activation occurs via a maturationally sensitive atypical pathway. Am J Physiol Lung Cell Mol Physiol 296: L296–L306
Baldwin AS 1996 The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14: 649–683
Hayden MS, Ghosh S 2008 Shared principles in NF-kappaB signaling. Cell 132: 344–362
Chen LF, Greene WC 2004 Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401
Ghosh S, May MJ, Kopp EB 1998 NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–260
Li Y, Zhang W, Mantell L, Kazzaz JA, Fein AM, Horowitz S 1997 Nuclear factor-kappa B is activated by hyperoxia but does not protect from cell death. J Biol Chem 272: 20646–20649
Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E 1996 Hyperoxia activates NF-kappa B and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J Immunol 157: 3902–3908
Dieperink HI, Blackwell TS, Prince LS 2006 Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res 59: 185–190
Franek WR, Morrow DM, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell L 2004 NF-kappa B protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med 37: 1670–1679
Franek WR, Horowitz S, Stansberry L, Kazzaz JA, Koo HC, Li Y, Arita Y, Davis JM, Mantell AS, Scott W, Mantell L 2001 Hyperoxia inhibits oxidant-induced apoptosis in lung epithelial cells. J Biol Chem 276: 569–575
D'Angio CT, LoMonaco MB, Johnston CJ, Reed CK, Finkelstein JN 2004 Differential roles for NF-kappa B in endotoxin and oxygen induction of interleukin-8 in the macrophage. Am J Physiol Lung Cell Mol Physiol 286: L30–L36
Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W 2001 Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol 62: 787–794
Breeze RG, Wheeldon EB 1977 The cells of the pulmonary airways. Am Rev Respir Dis 116: 705–777
Crapo JD, Barry BE, Foscue HA, Shelburne J 1980 Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143
Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA 2006 Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111
Barazzone-Argiroffo C, Pagano A, Juge C, Metrailler I, Rochat A, Vesin C, Donati Y 2003 Glucocorticoids aggravate hyperoxia-induced lung injury through decreased nuclear factor-kappa B activity. Am J Physiol Lung Cell Mol Physiol 284: L197–L204
Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr 1995 Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270: 283–286
Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M 1995 Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270: 286–290
Ray A, Prefontaine KE 1994 Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA 91: 752–756
Heck S, Bender K, Kullmann M, Gottlicher M, Herrlich P, Cato AC 1997 I kappaB alpha-independent downregulation of NF-kappaB activity by glucocorticoid receptor. EMBO J 16: 4698–4707
Aghai ZH, Kumar S, Farhath S, Kumar M, Saslow J, Nakhla T, Eydelman R, Strande L, Stahl G, Hewitt C, Nesin M, Rahman I 2006 Dexamethasone suppresses expression of Nuclear Factor-kappaB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatr Res 59: 811–815
Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE 2006 Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 355: 343–353
Franek WR, Chowdary YC, Lin X, Hu M, Miller EJ, Kazzaz JA, Razzano P, Romashko J, Davis JM, Narula P, Horowitz S, Scott W, Mantell LL 2002 Suppression of nuclear factor-kappa B activity by nitric oxide and hyperoxia in oxygen-resistant cells. J Biol Chem 277: 42694–42700
Kilpinen S, Henttinen T, Lahdenpohja N, Hulkkonen J, Hurme M 1996 Signals leading to the activation of NF-kappa B transcription factor are stronger in neonatal than adult T lymphocytes. Scand J Immunol 44: 85–88
Vancurova I, Bellani P, Davidson D 2001 Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res 49: 257–262
Yang G, Abate A, George AG, Weng YH, Dennery PA 2004 Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest 114: 669–678
Haddad JJ, Land SC 2000 O(2)-evoked regulation of HIF-1alpha and NF-kappaB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol 278: L492–L503
Bourbia A, Cruz MA, Rozycki HJ 2006 NF-kappaB in tracheal lavage fluid from intubated premature infants: association with inflammation, oxygen, and outcome. Arch Dis Child Fetal Neonatal Ed 91: F36–F39
Cao L, Liu C, Cai B, Jia X, Kang L, Speer CP, Sun B 2004 Nuclear factor-kappa B expression in alveolar macrophages of mechanically ventilated neonates with respiratory distress syndrome. Biol Neonate 86: 116–123
Cheah FC, Winterbourn CC, Darlow BA, Mocatta TJ, Vissers MC 2005 Nuclear factor kappaB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res 57: 616–623
Dohlen G, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD 2005 Reoxygenation of hypoxic mice with 100% oxygen induces brain nuclear factor-kappa B. Pediatr Res 58: 941–945
Toliver-Kinsky T, Rassin D, Perez-Polo JR 2002 NF-kappaB activity decreases in basal forebrain of young and aged rats after hyperoxia. Neurobiol Aging 23: 899–905
Levy DE, Darnell JE Jr 2002 Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3: 651–662
Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C 2005 Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 174: 7250–7256
Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA 2004 Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 113: 28–37
Nerlov C 2008 C/EBPs: recipients of extracellular signals through proteome modulation. Curr Opin Cell Biol 20: 180–185
Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE 2001 Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 155–161
Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M 2006 C/EBPalpha is required for lung maturation at birth. Development 133: 1155–1164
Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ 2004 Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801
Applegate LA, Luscher P, Tyrrell RM 1991 Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res 51: 974–978
Alam J, Cai J, Smith A 1994 Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5′ sequences are required for induction by heme or heavy metals. J Biol Chem 269: 1001–1009
Alam J, Camhi S, Choi AM 1995 Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem 270: 11977–11984
Lee PJ, Alam J, Wiegand GW, Choi AM 1996 Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA 93: 10393–10398
Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL 1999 Nrf2, a Cap‘n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078
Sun Z, Andersson R 2002 NF-kappaB activation and inhibition: a review. Shock 18: 99–106
Dennery PA, Lee CS, Ford BS, Weng YH, Yang G, Rodgers PA 2003 Developmental expression of heme oxygenase in the rat lung. Pediatr Res 53: 42–47
Kassovska-Bratinova S, Yang G, Igarashi K, Dennery PA 2009 Bach1 modulates heme oxygenase-1 expression in the neonatal mouse lung. Pediatr Res 65: 145–149
Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM 2003 Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696
Suttner DM, Dennery PA 1999 Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13: 1800–1809
Igarashi K, Sun J 2006 The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal 8: 107–118
Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A 1999 Role for NF-kappa B in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta 1448: 349–362
Suzuki Y, Nishio K, Takeshita K, Takeuchi O, Watanabe K, Sato N, Naoki K, Kudo H, Aoki T, Yamaguchi K 2000 Effect of steroid on hyperoxia-induced ICAM-1 expression in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 278: L245–L252
Vanden Berghe W, Vermeulen L, De Wilde G, De Bosscher K, Boone E, Haegeman G 2000 Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol 60: 1185–1195
Libermann TA, Baltimore D 1990 Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10: 2327–2334
Johnston CJ, Wright TW, Reed CK, Finkelstein JN 1997 Comparison of adult and newborn pulmonary cytokine mRNA expression after hyperoxia. Exp Lung Res 23: 537–552
Pitkanen O, Tanswell AK, Downey G, O'Brodovich H 1996 Increased Po2 alters the bioelectric properties of fetal distal lung epithelium. Am J Physiol 270: L1060–L1066
Otulakowski G, Rafii B, Bremner HR, O'Brodovich H 1999 Structure and hormone responsiveness of the gene encoding the alpha-subunit of the rat amiloride-sensitive epithelial sodium channel. Am J Respir Cell Mol Biol 20: 1028–1040
Rafii B, Tanswell AK, Otulakowski G, Pitkanen O, Belcastro-Taylor R, O'Brodovich H 1998 O2-induced ENaC expression is associated with NF-kappaB activation and blocked by superoxide scavenger. Am J Physiol 275: L764–L770
Haddad JJ, Collett A, Land SC, Olver RE, Wilson SM 2001 NF-kappaB blockade reduces the O2-evoked rise in Na+ conductance in fetal alveolar cells. Biochem Biophys Res Commun 281: 987–992
Richard K, Ramminger SJ, Inglis SK, Olver RE, Land SC, Wilson SM 2003 O2 can raise fetal pneumocyte Na+ conductance without affecting ENaC mRNA abundance. Biochem Biophys Res Commun 305: 671–676
Baines DL, Ramminger SJ, Collett A, Haddad JJ, Best OG, Land SC, Olver RE, Wilson SM 2001 Oxygen-evoked Na+ transport in rat fetal distal lung epithelial cells. J Physiol 532: 105–113
Hinata K, Gervin AM, Jennifer Zhang Y, Khavari PA 2003 Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene 22: 1955–1964
McGrath SA 1998 Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol 18: 179–187
O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM 1998 Accumulation of p21(Cip1/WAF1) during hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 19: 777–785
Corroyer S, Maitre B, Cazals V, Clement A 1996 Altered regulation of G1 cyclins in oxidant-induced growth arrest of lung alveolar epithelial cells. Accumulation of inactive cyclin E-DCK2 complexes. J Biol Chem 271: 25117–25125
Helt CE, Rancourt RC, Staversky RJ, O'Reilly MA 2001 p53-dependent induction of p21(Cip1/WAF1/Sdi1) protects against oxygen-induced toxicity. Toxicol Sci 63: 214–222
Helt CE, Staversky RJ, Lee YJ, Bambara RA, Keng PC, O'Reilly MA 2004 The Cdk and PCNA domains on p21Cip1 both function to inhibit G1/S progression during hyperoxia. Am J Physiol Lung Cell Mol Physiol 286: L506–L513
McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R 2004 The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol 30: 635–640
O'Reilly MA, Staversky RJ, Watkins RH, Reed CK, de Mesy Jensen KL, Finkelstein JN, Keng PC 2001 The cyclin-dependent kinase inhibitor p21 protects the lung from oxidative stress. Am J Respir Cell Mol Biol 24: 703–710
O'Reilly MA 2005 Redox activation of p21Cip1/WAF1/Sdi1: a multifunctional regulator of cell survival and death. Antioxid Redox Signal 7: 108–118
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Institutes of Health (RO-1 HL-58752) (P.A.D.) and Pediatric Scientist Development Program (K12-HD00850) (C.J.W.).
Rights and permissions
About this article
Cite this article
Wright, C., Dennery, P. Manipulation of Gene Expression by Oxygen: A Primer From Bedside to Bench. Pediatr Res 66, 5–10 (2009). https://doi.org/10.1203/PDR.0b013e3181a2c184
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a2c184
This article is cited by
-
Short-term perinatal oxygen exposure may impair lung development in adult mice
Biological Research (2020)
-
Biological effects of the oxygen molecule in critically ill patients
Journal of Intensive Care (2020)
-
Oxygen differentially affects the hox proteins Hoxb5 and Hoxa5 altering airway branching and lung vascular formation
Journal of Cell Communication and Signaling (2014)
-
Activation of STAT3 stimulates AHSP expression in K562 cells
Science China Life Sciences (2014)
-
Redox signaling in pathophysiology of hypertension
Journal of Biomedical Science (2013)