Abstract
Zinc has been recognized as an antioxidant with potential for chronic and acute effects. Oxidative damage produced by free radicals, including nitric oxide (NO), is responsible for certain types of intestinal malabsorption syndromes and diarrhea. Under physiologic or mildly stimulatory conditions for NO synthesis, the small intestine characteristically is in a proabsorptive state; however, an excessive production of NO triggers formation of cyclic nucleotides, which cause secretion and malabsorption. In this study, we hypothesized that low-molecular-weight, soluble zinc chelates could modulate the effects of induced NO excess on the small intestine. In vitro experiments demonstrated that zinc-citrate or zinc-histidine at ≥0.66 mM, as well as a known NO scavenger, 2-[carboxyphenyl]-4,4,4,4-tetramethylimidazoline-1-oxyl-3-oxide, at 2 μM, were effective at removing chemically generated NO. In vivo jejunal perfusions, conducted in healthy rats under anesthesia, showed that c-PTIO reduced the proabsorptive effects produced by 1 mM l-arginine, the precursor of NO. In a standard oral rehydration solution, 1 mM zinc-citrate partially reversed the antiabsorptive effects on potassium caused by an excess of NO generated from 20 mM l-arginine but did not alter sodium or water absorption. The data are consistent with the view that soluble zinc compounds incorporated into an oral rehydration solution may deserve further attention as a means to scavenge NO with fluids used for the treatment of chronic or acute diarrhea, especially in malnourished children who are often zinc deficient.
Similar content being viewed by others
Main
The importance of NO as a second messenger has become fully recognized in the last several years, especially in regard to endothelial tissue responses to endogenous stimuli (1). A reduction of NO synthesis from Arg in endothelia may lead to cell contraction, increased vascular protein leakage, and inflammation (2, 3). The latter may be linked to free radical formation. The severity of chronic ileitis has been associated with the extent of NO synthesis by inducible NO synthase (iNOS) in the intestine (4). This may play a role on the integrity of the mucosal barrier, compromised in that condition. Proinflammatory cytokines up-regulate iNOS, as experimentally shown in chemically induced colitis injury (5), after injection of IL-1α(6) and in cancer patients receiving IL-2 (7). Animal and human studies support the view that zinc has anti-inflammatory properties. In rats with 2,4,6-trinitrobenzenesulfonic acid-induced colitis, zinc sulfate enemas produced a decrease in the lesion area and in other markers of inflammation (8). In another context, zinc ions have been considered an important anti-inflammatory factor because they appear to block human rhinovirus docking on intercellular adhesion molecule-1 (ICAM-1) on somatic cells, thus interrupting the inflammatory process (9). Experiments on rats injected with lipopolysaccharide (LPS) to induce inflammation showed that endogenous zinc inhibited LPS or IL-1-induced NO formation, as well as reduced the activity of smooth muscle cell NOS. These findings were attributed to the anti-inflammatory activity of zinc (10).
Inhibition of NO synthesis may produce a “dysfunctional” epithelial barrier with partial loss of protection against the transcellular passage of potentially noxious antigens, as well as bacterial translocation (11). NO synthesis in intestinal tissue is regulated at two levels. A constitutive NOS (cNOS) provides a baseline level, but the regulation of NO production is largely modulated by iNOS. NO activates formation of cGMP, which in turn enhances synthesis of protein kinase C. Phosphorylation and dephosphorylation of this enzyme modifies myosin light chain structure leading to contraction and relaxation of interepithelial cell junctions and increased permeability of the mucosal barrier. In addition, protein kinase C acts on transmembrane transporters resulting in leakage of chloride, concurrent sodium loss, and diarrhea. In other organs, zinc added to a cardioplegic solution as a l-histidine (His) complex reduced postischemic reperfusion injury in rats, and acted as a myocardial protector in pigs (12, 13).
Vallee (14) provided an explanation for attributing antioxidant or free radical scavenger roles to soluble organometallic zinc complexes, arguing that a stable zinc-ligand chelate may acquire the necessary electronic configuration to exhibit redox properties, although it is not, per se, a transition element. The present studies set out to validate the hypothesis that low molecular weight zinc-ligand complexes could act as NO scavengers; first in vitro, using a chemical NO generating system, and subsequently in vivo, by jejunal perfusion of ORS. Because ORS are extensively used in the treatment of diarrheal diseases of diverse etiologies, and zinc deficiency is associated with chronic diarrheal disease of infancy (15, 16), the outcome of this work may have implications for the formulation of ORS.
METHODS
Experiments in vitro.
To investigate whether low molecular weight zinc chelates act as NO scavengers, a chemical NO generating system based on the reduction of nitrite with iodide under controlled pH (adapted from ref. 17) was exposed to increasing concentrations of zinc chelates with His (1:2) and citrate (1:1), using the conversion of oxyhemoglobin to methemoglobin as an indicator of NO formation. A human red cell hemolysate was used as an oxyhemoglobin source, diluted so that the initial absorbance at 576 nm was approximately 0.150. Disappearance of oxyhemoglobin was followed spectrophotometrically for 2 min. The test solution contained a N-2-hydroxyethylpiperazine-N-2′-ethanesulfonic acid (HEPES)-Na buffer, pH 8.0, at a final concentration of 1 mM and NaI at 2 mM. The reaction was initiated by the addition of a NaNO2 solution to yield a final concentration of 2 mM. c-PTIO (Calbiochem, La Jolla, CA, U.S.A.) at 0.5 and 2 μM concentration was used as a reference known NO scavenger (18, 19).
Experiments in vivo.
The role of zinc as an NO scavenger in vivo was investigated using a jejunal perfusion procedure, as applied in previous studies (20, 21). In brief, 60–80 g Sprague-Dawley male rats (Taconic Farms., Germantown, NY, U.S.A.) were acclimatized to the conditions of the animal facility for at least 48 h, fasted overnight, and anesthetized with urethane (1.3 g/kg, ip). An intestinal segment immediately distal to the ligament of Treitz was cannulated, rinsed with saline, and perfused with the solutions described below using a peristaltic pump (Model 1203, Harvard Instruments, Holliston, MA, U.S.A.) for 3 h at 10–12 mL/h. An initial 1 h of perfusion was required to achieve steady state absorption rates, and effluents obtained during this time were discarded. Afterward, five 15-min fractions were collected for analysis, as described below. Two sets of comparative perfusions were conducted. In the first one, the goal was to determine to what extent putative changes in the rates of NO production by either low concentration of added Arg (1 mM), or c-PTIO, could modulate water, sodium, and glucose absorption, in vivo. The basal fluid was an ORS containing 60 mM sodium chloride, 111 mM glucose, 20 mM potassium chloride, and 10 mM trisodium citrate. In the second set of experiments, the ORS described above had as additives either Arg (20 mM), zinc acetate (1 mM), or both together. For comparison, 2 μmol/L c-PTIO added to 20 mM Arg was used. If not otherwise indicated, chemicals were purchased from Sigma Chemical (St. Louis, MO, U.S.A.). Tritiated water (2 μCi/L = 74 MBq/L; PerkinElmer Life Science Products, Boston, MA, U.S.A.) was added to all solutions to quantify unidirectional water movement: its disappearance from effluents was used to determine water influx. Net water absorption was computed as the difference in weight of solutions entering and leaving the perfused intestinal segment. Water efflux, or secretion, was calculated by difference between influx and net water absorption. I/E, a sensitive index of changes in unidirectional fluid movement (21), was also tabulated. Sodium and potassium were assayed by atomic absorption spectrophotometry (SpectrAA 10; Varian Instruments, Sunnyvale, CA, U.S.A.). Beta emission of tritium was quantitated with a scintillation counter (Tri-Carb 1900TR, Packard Instrument Co., Meriden, CT, U.S.A.) calibrated with external standards. Absorption rates were expressed as nanomole (or μL) per minute per centimeter. Results for the 15-min fractions collected for every animal were averaged and subsequently used to calculate fluid and solute transport rates for each treatment. Figures and tables present means and SEM.
The in vivo studies were approved by the institutional animal utilization and care committee.
Statistical analysis.
Comparisons among groups were by one-way ANOVA and posthoc Tukey test. If data were not normally distributed, the Kruskal-Wallis ANOVA on ranks test, with multiple comparisons versus a control group, was carried out. A computer program (SigmaStat, SPSS Science, Chicago, IL, U.S.A.) was used for these calculations. The threshold of significance was 0.05.
RESULTS
Experiments in vitro.
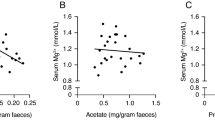
Concentrations of zinc ≥0.66 mM significantly altered disappearance of oxyhemoglobin, and in the 1–2 mM range reduced methemoglobin synthesis by at least 50%. This was observable when zinc was tested in either a citrate buffer (Fig. 1A) or chelated with His (Fig. 1B). Zinc concentration–dependent changes were more sharply defined in the citrate buffer experiments. The effects of low molecular weight zinc chelates were comparable to those obtained under similar conditions with c-PTIO, albeit at a lower concentration (Fig. 1C).
Time course of oxyhemoglobin disappearance in an in vitro NO generating system in the presence of variable concentrations of zinc in a 1:1 citrate buffer (A) or in a 2:1 His buffer (B). The NO scavenging effect of two concentrations of c-PTIO is presented for comparative purposes (C). The error for each point represents the SEM.
Experiments in vivo.
The addition of 1 mM Arg to ORS increased sodium absorption, although net water and glucose absorption were unaltered (Fig. 2). Addition of 2 μM c-PTIO to ORS had no effect on either water, sodium, or glucose absorption. When the combination of 1 mM Arg and 2 μM c-PTIO was added to the ORS, there was reduced net water and glucose absorption. Differences observed in water absorption could be associated with an increase in water efflux and a consequent reduction in the I/E ratio (Table 1).
Net water, sodium, and glucose absorption rates during perfusion under anesthesia of an ORS in the absence or presence of either 1 mM Arg or 2 μM c-PTIO and the combination of both. The bars represent means ± SEM. In each graph, bars not sharing a superscript are different (p < 0.05). The number of animals in each group is given in Table 1.
In perfusions where 20 mmol/Arg was added to ORS, sodium absorption was significantly reduced by Arg, and net water absorption was diminished, although to a lesser extent (Fig. 3). Changes in water absorption were clearly revealed by changes of the I/E ratio, which was decreased by 20 mmol/Arg, and 20 mM Arg plus 1 mM zinc (Table 2). Potassium absorption was increased by zinc, although when Arg and zinc were perfused together there was no effect. Glucose absorption remained unaltered in all treatments (Fig. 3).
Net water, sodium, and glucose absorption rates, under conditions described in the text, with a standard ORS, or the same solution containing either 20 mM Arg, 1 mM zinc, both additives, or 20 mM Arg plus 2 μM c-PTIO. The bars represent means ± SEM. In each graph, bars not sharing a superscript are different (p < 0.05). The number of animals in each group is given in Table 2.
DISCUSSION
We have obtained evidence that complexes of low molecular weight ligands and zinc can hasten the removal or inactivation of nascent NO in vitro. The mechanism involved is possibly an accelerated conversion of NO to nitrite and nitrate, the normal metabolic stable end points of NO under aerobic conditions. This is reflected in a slowdown of oxyhemoglobin conversion to methemoglobin, as followed by changes in their absorption characteristics. Under the conditions described, the His-zinc chelate appeared to be somewhat more effective than citrate-chelated zinc. This was consistent with the contributory scavenging effects obtained with His buffer alone, when zinc was omitted.
The results of the in vitro experiments prompted the carrying out of the following step, that is, to investigate whether such property could be translated to an in vivo situation, resulting in an improvement of the absorptive capacity of the small intestine under either proabsorptive or moderately secretory conditions. This evaluation was monitored by measuring absorption rates during perfusions with ORS. To have a frame of reference for the effects of zinc complexes on intestinal transport, the proven NO scavenger c-PTIO was tested in the same ORS at a concentration shown in vitro, as well as in other studies (18), to be effective for the removal of NO. This potential was evaluated in the presence or absence of 1 mM Arg, a concentration of this amino acid shown to increase absorption of water and electrolytes (21), presumably by generation of proabsorptive amounts of additional NO in the small intestinal mucosa (22). This hypothesis was upheld because c-PTIO negated the proabsorptive effects of low-dose Arg (Fig. 2, Table 1). c-PTIO in combination with Arg further depressed water absorption rates. This effect on water influx and glucose absorption was manifested to a greater extent than when c-PTIO was perfused alone. A possible explanation may be that the imidazoline and/or the oxyl-oxide groups of c-PTIO react with the guanidino or the amino groups of Arg in the solution, thus reducing the proabsorptive action of the latter. Based on these findings, it could be expected that other NO scavengers, such as low molecular weight zinc chelates, could also modulate the effects of NO in vivo.
The approach followed in this study regarding the use of Arg at different concentrations is based on previous work showing that the rate at which NO is synthesized in the enterocyte by iNOS is regulated by Arg concentration, the enzyme substrate. Arg administration, either intravenous or oral, modulates small intestinal physiology. In perfused rat jejunum, low concentrations of Arg (1–2 mM) were shown to have proabsorptive effects, increasing net water and sodium absorption. At a higher Arg concentration (20 mM), a small secretory effect could be measured (21). These concentration-dependent pro and antiabsorptive actions of Arg, putatively related to NO production, have subsequently been confirmed (23).
Absorption of glucose from solutions containing sodium and glucose in near equimolar concentration (90 and 111 mM, respectively), such as the standard ORS we used, is largely mediated by SGLT1, the Na-dependent glucose transporter of the small intestinal apical mucosa (24). In addition, at the relatively high concentration of glucose introduced, transmembrane diffusion may account for a significant fraction of glucose uptake (25). Both transport mechanisms may offset the NO generating capacity provided by Arg in situ. The inclusion of 20 mM Arg into the ORS reduced net water absorption (Fig. 3), an effect made more prominent in the significant reduction of the I/E ratio (Table 2) as a consequence of divergent changes in water influx and efflux. Addition of 1 mM zinc increased potassium absorption, apparently in response to a greater rate of water influx. However, at the concentration added, zinc did not alter the effects produced by 20 mM Arg. The minimal changes observed in absorption rates and unidirectional fluid movement may the result of the dominant role of the sodium-linked glucose absorption process, driven by SGLT1 (26), which is intact in normal animals, such as those used in this study. In the presence of a high glucose concentration, mechanisms other than active transport may play an important role, and diffusion resulting from a concentration gradient could become predominant and mitigate the prosecretory effects of excess Arg. An increased potassium absorption may be beneficial in protracted gastrointestinal disease with diarrhea and vomiting. Hence, zinc added at a 1 mM concentration might have a positive effect on intestinal function and may also provide supplemental, easily absorbable zinc. Although millimolar zinc concentrations in vitro were comparable to a micromolar concentration of c-PTIO in their effect as NO scavengers, the same effectiveness was not achieved in vivo. It is possible that low molecular weight zinc chelates are absorbed, but there is no evidence that this is the case for c-PTIO, which has an anionic conformation and should be far less absorbable than zinc complexes; thus, its local action may be more pronounced.
There have been numerous studies linking diarrheal disease with zinc deficiency. This connection was first inferred from reports of low plasma zinc in children with diarrhea (27, 28). More recently, it has been shown that serum and rectal biopsy zinc concentrations are decreased in children and infants with chronic diarrhea (29). Also, considerable zinc losses have been reported in children with acute dehydrating diarrhea (30). These findings have elicited studies of zinc supplementation in children with diarrhea (15, 31–34). Zinc supplementation may be responsible for accelerating recovery from diarrhea, especially in infants and children with overt or subclinical zinc deficiency. Meta-analyses of the numerous clinical trials carried out in several continents support the benefits of zinc supplementation (35, 36). However, the optimal amount of zinc for supplementation has not been well established inasmuch as a greater mortality rate and response failure occurred in malnourished children given 6 mg/kg/d, compared with those receiving 1.5 mg/kg/d (37), possibly because of a depressed monocyte function (38). However, there is consensus that short-term high doses of zinc are safe (39). A large amount of ingested zinc may reduce copper absorption; this may be especially relevant early in life (40). Nevertheless, ORS are intended for short-term use and prompt nutritional rehabilitation; therefore, even a relatively high zinc concentration in the ORS, 1 mM, as used in this experiment, may not be excessive. The choice of this zinc concentration was based on the results of the in vitro experiments that showed a definite effect on NO removal.
Zinc consideration as an antioxidant stems from its presence in superoxide dismutase (SOD), an enzyme with a major role as scavenger of free radicals in the cytoplasm of many types of cells and in the extracellular space (41). Cu/Zn-SOD converts the superoxygen free radical ·O2− to hydrogen peroxide, which is further decomposed by catalase into water and oxygen (42). In addition, zinc is considered an antioxidant because of its role as an intramolecular stabilizer, preventing oxidation of the thiol group of cysteine in proteins and the formation of disulfide bonds (43). The evidence presented here that low molecular weight zinc chelates can delay the oxidation of oxy- to methemoglobin substantiates their antioxidant properties, which have already been documented under other conditions (44–47).
Another aspect of the antioxidant characteristics associated with zinc in the intestine relates to its interaction with intracellular thionein (T) to form metallothionein (MT). Element-binding transcription factors induce T, which subsequently binds zinc to form zinc-MT, the protein that most tightly binds zinc in the organism. MT has been considered a thermodynamic “sink” for this element (48, 49). Slow exchanges of zinc-MT with other disulfide molecules, such as oxidized glutathione (GSSG), create a moderately reducing intracellular redox pair GSH/GSSG to which some of the antioxidant properties attributed to zinc can be traced.
CONCLUSION
In conclusion, the addition of zinc directly to an ORS may operate in several possible ways: a) by providing a readily absorbable form of zinc to zinc-depleted tissues, where zinc may also act as an antiinflammatory agent (27–29, 31–36); 2) by introducing antioxidant organometallic complexes (42, 43, 45–47), capable of inducing MT synthesis in the enterocyte (48, 49); or c) by scavenging NO diffusing out of the enterocyte, especially under conditions of increased NO synthesis, thus creating a NO gradient toward the intestinal lumen.
Abbreviations
- Arg, l-arginine c-PTIO:
-
2-[carboxyphenyl]-4,4,4,4-tetra-methylimidazoline-1-oxyl-3-oxide
- I/E:
-
ratio between water influx and efflux
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase (EC 1.14.13.39)
- ORS:
-
oral rehydration solution
References
Ignarro LJ, Cirino G, Casini A, Napoli C 1999 Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol 34: 879–886
Clancy RM, Abramson SB 1995 Nitric oxide: a novel mediator of inflammation. Proc Soc Exp Biol Med 210: 93–101
Wu G, Meininger CJ 2000 Arginine nutrition and cardiovascular function. J Nutr 130: 2626–2629
Miller MJ, Sadowska-Krowicka H, Chotinuaremol S, Kakkis JL, Clark DA 1993 Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther 264: 11–16
Southey A, Tanaka S, Murakami T, Miyoshi H, Ishizuka T, Sugiura M, Kawashima K, Sugita T 1997 Pathophysiological role of nitric oxide in rat experimental colitis. Int J Immunopharmacol 19: 669–676
Cui L, Okada A 2000 Nitric oxide and manifestations of lesions of skin and gastrointestinal tract in zinc deficiency. Curr Opin Clin Nutr Metabol Care 3: 247–252
Locker GJ, Koffler J, Stoiser B, Wilfing A, Wenzel C, Wogerbauer M, Steger GG, Zielinski CC, Mader R, Burgmann H 2000 Relation of pro- and anti-inflammatory cytokines and the production of nitric oxide in patients receiving high-dose immunotherapy with interleukin-2. Eur Cytokine Netw 11: 391–396
Chen BW, Wang HH, Liu JX, Liu XG 1999 Zinc sulphate solution enema decreases inflammation in experimental colitis in rats. J Gastroenterol Hepatol 14: 1088–1092
Novick SG, Godfrey JC, Pollack RL, Wilder HR 1997 Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Med Hypotheses 49: 347–357
Abou-Mohamed G, Papapetropoulos A, Catravas JD, Caldwell RW 1998 Zn2+ inhibits nitric oxide formation in response to lipopolysaccharides: implications in its antiinflammatory activity. Eur J Pharmacol 341: 265–272
Alican I, Kubes P 1996 A critical role for nitric oxide in intestinal barrier functions and dysfunction. Am J Physiol 270: G225–G237
Powell SR, Hall D, Aiuto L, Wapnir RA, Teichberg S, Tortolani AJ 1994 Zinc improves postischemic recovery of the isolated rat heart through inhibition of oxidative stress. Am J Physiol 266: H2497–H2507
Powell SR, Nelson RL, Finnerty JM, Alexander D, Pottanat G, Kooker K, Schiff RJ, Moyse J, Teichberg S, Tortolani AJ 1997 Zinc-bis-histidinate preserves cardiac function in a porcine model of cardioplegic arrest. Ann Thorac Surg 64: 73–80
Vallee BL 1995 The function of metallothionein. Neurochem Int 27: 23–33
Folwaczny C 1997 Zinc and diarrhea in infants. J Trace Elem Med Biol 11: 116–122
Fuchs GJ 1998 Possibilities for zinc in the treatment of acute diarrhea. Am J Clin Nutr 68: 480S–4803S
Brauer G 1963 Handbook of Preparative Inorganic Chemistry, 2nd Ed. Academic Press, New York, 485–487.
Akaike T, Maeda H 1996 Quantitation of nitric oxide using 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO). Methods Enzymol 268: 211–221
Pfeiffer S, Leopold E, Hemmens B, Schmidt K, Werner ER, Mayer B 1997 Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med 22: 787–794
Go JT, Harper RG, Sia CG, Teichberg S, Wapnir RA 1994 Oral rehydration solutions: increased water and sodium absorption by addition of a viscosity-enhancing agent in a rat model of chronic osmotic diarrhea. J Pediatr Gastroenterol Nutr 19: 410–416
Wapnir RA, Wingertzahn MA, Teichberg S 1997 l-arginine in low concentration improves rat intestinal water and sodium absorption from oral rehydration solutions. Gut 40: 602–607
Wingertzahn MA, Teichberg S, Wapnir RA 1998 Jejunal nitric oxide levels are reduced by gum arabic (GA). J Am Coll Nutr 17: 509( abstr)
Schirgi-Degen A, Beubler E 1998 Proabsorptive properties of nitric oxide. Digestion 59: 400–403
Loo DDF, Zeuthen T, Chandy G, Wright EM 1996 Cotransport of water by the Na+-glucose cotransporter. Proc Natl Acad Sci U S A 93: 13367–13370
O'Rourke M, Shi X, Gisolfi C, Schedl H 1995 Effect of absorption ofd-glucose and water on paracellular transport in rat duodenum. Am J Med Sci 309: 146–151
Shirazi-Beechey SP 1995 Molecular biology of intestinal glucose transport. Nutr Res Rev 8: 27–41
Naveh Y, Lightman A, Zinder O 1982 Effect of diarrhea on serum zinc concentrations in infants and children. J Pediatr 101: 730–732
Sarker SA, Rahaman MM, Ali A, Hossain S, Alam AN 1985 Prolonged depression of serum zinc concentrations in children following post-measles diarrhoea. Hum Nutr Clin Nutr 39: 411–417
Sachdev HP, Mittal NK, Yadav HS 1990 Oral zinc supplementation in persistent diarrhoea in infants. Ann Trop Paediatr 10: 63–69
Ruz M, Solomons NW 1990 Mineral excretion during acute, dehydrating diarrhea treated with oral rehydration therapy. Pediatr Res 27: 170–175
Sachdev HP, Mittal NK, Mittal SK, Yadav HS 1988 A controlled trial on utility of oral zinc supplementation in acute dehydrating diarrhea in infants. J Pediatr Gastroenterol Nutr 7: 877–881
Roy SK, Behrens RH, Haider R, Akramuzzaman SM, Mahalanabis D, Wahed MA, Tomkins AM 1992 Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr 15: 289–296
Sazawal S, Black RE, Bhan MK, Jalla S, Sinha A, Bhandari N 1997 Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea—a community-based, double-blind, controlled trial. Am J Clin Nutr 66: 413–418
Brown KH, Peerson JM, Allen LH 1998 Effect of zinc supplementation on children's growth: a meta-analysis of intervention trials. Bibl Nutr Dieta 54: 76–83
Zinc Investigators' Collaborative Group 1999 Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr 135: 689–697
Zinc Investigators' Collaborative Group 2000 Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized trials. Am J Clin Nutr 72: 1516–1522
Doherty CP, Kashem Sarkar MA, Shakur MS, Ling SC, Elton RA . Cutting WA 1998 Zinc and rehabilitation from severe protein-energy malnutrition: higher-dose regimens are associated with increased mortality. Am J Clin Nutr 68: 742–748
Schlesinger L, Arevalo M, Arredondo S, Lönnerdal B, Stekel A 1993 Zinc supplementation impairs monocyte function. Acta Paediatr 82: 734–738
Shankar AH, Prasad AS 1998 Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68( suppl): 447S–463S
Sandstead HH 1995 Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am J Clin Nutr 61: 621S–624S
Bray TM, Bettger WJ 1990 The physiological role of zinc as an antioxidant. Free Radic Biol Med 8: 281–291
Leung FY 1998 Trace elements that act as antioxidants in parenteral micronutrition. J Nutr Biochem 9: 304–307
Powell SR 2000 The antioxidant properties of zinc. J Nutr 130: 1447S–1454S
Hegenauer J, Saltman P, Fairchild R, Halasz NA 1991 Improved function of reperfused rabbit kidney following administration of zinc histidine. J Trace Elem Exp Med 4: 103–107
Afanas'ev IB, Suslova TB, Cheremisina ZP, Abramova NE, Korkina LG 1995 Study of antioxidant properties of metal aspartates. Analyst 120: 859–862
Tons C, Klosterhalfen B, Klein HM, Raul HM, Anurov M, Oettinger A, Schumpelick V 1997 Induction of heat shock protein 70 (HSP70) by zinc bis (DL-hydrogen aspartate) reduces ischemic small bowel tissue damage in rats. Langebecks Arch Chir 382: 43–48
Maret W, Vallee BL 1998 Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A 95: 3478–3482
Jacob C, Maret W, Vallee BL 1998 Control of zinc transfer between thionein, metallothionein and zinc proteins. Proc Natl Acad Sci U S A 95: 3489–3494
Maret W 2000 The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130: 1455S–1458S
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported, in part, by a grant from the Nutricia Foundation, The Netherlands.
Rights and permissions
About this article
Cite this article
Wingertzahn, M., Rehman, K., Altaf, W. et al. Zinc as a Potential Enteroprotector in Oral Rehydration Solutions: Its Role in Nitric Oxide Metabolism. Pediatr Res 53, 434–439 (2003). https://doi.org/10.1203/01.PDR.0000049465.73687.4D
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000049465.73687.4D
This article is cited by
-
Roles of Zinc in the Pathophysiology of Acute Diarrhea
Current Infectious Disease Reports (2012)
-
The use of oral rehydration solutions in children and adults
Current Gastroenterology Reports (2004)