Abstract
Gitelman disease was diagnosed in two unrelated children with hypokalemic metabolic alkalosis and growth failure (a boy and a girl aged 7 mo and 9.5 y, respectively, at clinical presentation) on the basis of mutations detected in the gene encoding the thiazide-sensitive NaCl cotransporter of the distal convoluted tubule. GH deficiency was demonstrated by specific diagnostic tests in both children. Hypertonic saline infusion tests showed a partial vasopressin deficiency in the girl and delayed secretion of this hormone in the boy. Magnetic resonance imaging revealed an empty sella in both cases. Up to now, hypomagnesemia and hypocalciuria have been considered obligatory criteria for the diagnosis of Gitelman disease; however, our two patients had hypomagnesia and hypocalciuria in less than half the determinations. GH replacement treatment was associated with a good clinical response in both children. It appears that these cases represent a new phenotype, not previously described in Gitelman disease, and that the entity may be considered a new complex hereditary renal tubular-pituitary syndrome.
Similar content being viewed by others
Main
Molecular studies have recently demonstrated that the hereditary renal tubular disorder characterized by hypomagnesemia-hypokalemia with hypocalciuria known as Gitelman disease(1) is caused by mutations in the gene encoding the TSC (NCCT or SLC12A3) of the distal convoluted tubule(2,3). Clinically, these patients are normotensive, with tetanic crises appearing during childhood or later and/or muscular weakness, or they may be completely asymptomatic(1,4–6). Growth failure, although described in some cases, was not considered one of the classic findings of the disease(5). Furthermore, only one case with GH deficiency has been reported(7). Hypokalemia associated with metabolic alkalosis, hypomagnesemia of renal tubular origin, hypocalciuria, hyperreninemia, and normal GFR are the most important biochemical findings(1,4–6). Here we describe two children with Gitelman diseased, definitively diagnosed by molecular evaluation, who presented a new phenotype characterized by GH deficiency, disturbances in vasopressin secretion, empty sella, and normal values of serum magnesium and urinary calcium excretion in more than half the determinations.
CASE REPORTS
Case 1. This girl was born at term with a birth weight of 2800 g. No polyhydramnios was reported. Her family history was negative for renal, endocrinologic, and cardiovascular diseases. She had no disease during the first 9.5 y of life and complained only of nocturia of some months' duration. At this time she was admitted to an emergency service with abdominal pains. Her blood pressure was normal (106/60 mm Hg). Her height was 121 cm (-2.1 height SD for chronologic age) (Fig. 1), and her weight for height index was 93%. Bone age according to Tanner Whitehouse method (TW2) was 7.0 y. Abdominal ultrasound evaluation, including the kidneys, was normal. Biochemical tests revealed severe hypokalemia (serum potassium, 2.3 mM), and KCl infusion i.v. (90 mmol/d). was started. Two days after admission, the abdominal pain disappeared and the KCl infusion was stopped. However, hypokalemia (serum potassium, 2.3-3.2 mM) persisted, associated with high FE of potassium (22.8-54%) and metabolic alkalosis (venous pH, 7.43; plasma HCO3, 29 mM). GFR expressed as creatinine clearance was normal (119 mL·min-1·1.73 m-2), as were serum sodium (139 mM) and total calcium (2.45 mM). Serum magnesium was normal at admission (0.83 mM), and normal or slightly decreased (0.88-0.63 mM) in the following 2 wk with an FEMg of 4.8-6.3%. During this period, serum magnesium was normal (>0.75 mM) in seven of 10 determinations. Serum chloride was 97 mM and FECl was high (3.3%). Urinary calcium excretion, evaluated as the molar urinary calcium/creatinine ratio, was normal (0.49) at the first evaluation and normal to low (0.32-0.09) later on. Hypocalciuria (molar urinary calcium/creatinine < 0.20)(5) was observed in four of 10 determinations. Proteinuria, hemoglobinuria, and glucosuria were not detected. Other tests demonstrated hyperreninemia (24.6 ng·mL-1·h-1) and hyperaldosteronism (537 pg/mL). Maximal urinary osmolality obtained after 20 µg of intranasal desmopressin was 730 mosm/kg. Glucose tolerance test was normal. The most probable diagnosis was Gitelman disease even though some results (i.e. serum magnesium and urinary calcium excretion) were normal in most determinations.
GH tests, performed to investigate growth retardation, revealed GH deficiency, as shown by GH nocturnal secretion (mean GH concentration, 1.2 ng/mL; no peaks > 10 ng/mL in a 12-h profile including 28 samples) and GH response to clonidine stimulation (GH peak, 6 ng/mL). Serum IGF-I was low (89 ng/mL) with respect to our laboratory range and controls of the same age and sex (89-626 ng/mL). A partial deficiency of vasopressin secretion was diagnosed after a hypertonic saline infusion test (see below).
Other hormonal investigations included free T3 (5.0 pg/mL), free T4 (12.5 pg/mL), TSH (2.11 µU/mL), prolactin (3.6 ng/mL), LH (0.1 mIU/mL), FSH (1.6 mIU/mL), testosterone (< 0.1 ng/mL), 17-α-OH-progesterone (1.0 ng/mL), cortisol (146 ng/mL), insulin (19.7 µIU/mL), and immunoreactive PTH (19 pg/mL). All these results were in the reference ranges for age.
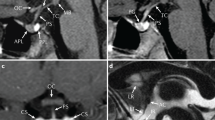
Magnetic resonance imaging demonstrated a sella turcica of normal size, small hypophysis, posterior hypophysis in the sella turcica but on the right side, and normal hypothalamus and chiasma. Intrasellar arachnoid evaginations were observed, indicating an empty sella.
At 9.8 y of age (height still 121 cm, -2.4 SD), the patient started GH treatment of 0.8 IU/kg weekly in five doses. After 1 y, height velocity increased to 10.9 cm/y with a recovery of + 1.4 height SD for chronologic age with respect to pretreatment. No side effects have occurred up to now.
Intranasal desmopressin (10 µg/d) was also administered, with complete resolution of nocturia after a few days of treatment.
In the 1-y treatment period, in which KCl supplementation was also prescribed (3 mmol·kg-1·d-1), serum magnesium ranged between 0.74 and 1.15 mM and hypokalemia persisted (serum potassium, 2.5-3.5 mM) with an FEK of 24-43%. Molar urinary calcium/creatinine ratio was 0.17-0.25.
Case 2. A postmature infant was born at 43 wk gestation by operative delivery with application of vaccum extractum, with a birth weight of 3350 g and Apgar score of 8. Abnormal uterine activity had been reported since the sixth month of pregnancy. No polyhydramnios was reported. The family history was negative for renal, endocrinologic, and cardiovascular diseases. At 7 mo of age, the infant was admitted to a pediatric ward for excessive perspiration, anorexia, constipation, and polyuria. Physical examination was remarkable for his poor general condition, delayed psychomotor development, and hypotonia. His weight was 5.7 kg (weight for height index, 93%) and height 61.5 cm (-3.4 SD). Blood pressure was normal (85/60 mm Hg). The most important biochemical findings were hypokalemia (serum potassium, 3.1 mM) and metabolic alkalosis (venous pH, 7.51; plasma HCO3, 36.4 mM). Serum creatinine was in the reference range for age (0.3 mg/dL), were serum sodium (135 mM), chloride (98 mM), total calcium (2.58 mM), and magnesium (0.96 mM). Calciuria was in the normal range for age (0.08 mmol·kg-1·d-1). No proteinuria, hemoglobinuria, or glucosuria were detected. Other tests demonstrated marked hyperreninemia (250 ng·mL-1·h-1) and hyperaldosteronism (serum aldosterone 4.300 pg/mL). Thyroid hormone levels, sweat test, and oral glucose tolerance test were normal as was abdominal ultrasound scan. A diagnosis of Bartter syndrome was hypothesized, and treatment with spironolactone (1 mg·kg-1·d-) and KCl (5 mEq·kg-1·d-1) was started. Plasma pH and serum chloride levels subsequently normalized, whereas potassium levels remained between 3.0 and 3.5 mM.
Growth failure (Fig. 2) persisted, and at age 3.5 y, his height was 87 cm (-2.74 SD for chronologic age), height velocity was 3.5 cm/y (-3 SD for chronologic age), weight was 11.7 kg (weight for height index, 88%), and bone age (TW2-RUS) was 1.8 y. GH secretion was evaluated by insulin tolerance, arginine, and clonidine tests in which the hormone always peaked at values below the lower normal limit (8 ng/mL). GH deficiency was diagnosed.
GH treatment was started at the dose of 0.7 IU/kg weekly. The growth rate increased from 6 cm/y to 11 and 9 cm/y in the subsequent 2 y, respectively (Fig. 2). Inasmuch as hypokalemia persisted (serum potassium, 2.7-3.4 mM), indomethacin was introduced (2 mg·kg-1·d-1) when the child was 6.3 y old. The combined GH and indomethacin treatment further improved growth, and at age 7.2 y, his height was 118 cm (-0.75 SD). Indomethacin treatment was continued, but GH treatment was stopped to evaluate spontaneous growth. At age 10 y, the boy was referred to the Milan University Department of Pediatrics for clinical and diagnostic re-evaluation. His height was 140 cm (+0.60 SD) (Fig. 2) and weight was 40 kg (weight for height index, 124%). He showed signs of anticipated puberty as increased testicular volume (6 mL), advanced bone age (+1.5 y with respect to chronologic age), and midpubertal testosterone concentration (four samples in early morning = 2.0 ng/mL). The child presented speech problems, uncertain gait, and difficulty in making finger movements. The karyotype was normal (46,XY) and a search for fragile X chromosome was negative. At that time he was receiving spironolactone (3.7 mg·kg-1·d-), KCl (3 mEq·kg-1·d-1), and indomethacin (3.7 mg·kg-1·d-1). Biochemical tests confirmed severe hypokalemia (serum potassium, 2.4 mM) with high FEK (56%) and metabolic alkalosis (venous pH, 7.47; plasma HCO3, 32 mM). Other tests revealed hypochloremia (90 mM) associated with inappropriately increased FECl (1.5%), hyperreninemia (32 ng·mL-1·h-1), and hyperaldosteronism (1539 pg/mL); normal to slightly low serum magnesium (0.67-0.80 mM) with FEMg of 5.0%; and normal to low molar urinary calcium/creatinine ratio (0.30-0.15). In six of 10 determinations, serum magnesium and urinary calcium values were normal. GFR expressed as creatinine clearance was also normal (114 mL·min-1·1.73 m-2). Urinary concentrating ability was abnormal as suggested by desmopressin test; the maximum urinary value was 480 mosm/kg. A hypertonic saline test (see below) demonstrated delayed vasopressin secretion. At the same time, an evaluation of GH secretion showed a pathologic nocturnal profile with a mean GH concentration of 1.2 ng/mL and absence of peaks > 10 ng/mL and a pathologic response to GH releasing hormone + pyridostigmine (maximum GH peak, 16 ng/mL). Other endocrinologic determinations were normal: FSH, 13 mU/mL; basal LH, 2.7 mU/mL (peak, 7.5 mU/mL); prolactin, 10.3 ng/mL; cortisol, 87 ng/mL; TSH, 2.6 mU/mL; free T3, 5.6 pg/mL; free T4, 12.7 pg/mL; and IGF-I, 539 ng/mL (normal range for our laboratory for pubertal boys, 192-786 ng/mL).
Magnetic resonance imaging of the pituitary gland showed sella turcica of normal size but partly occupied by arachnoid evaginations. This picture was compatible with an empty sella. An enlargement of the supratentorial ventricles was also observed.
Because of the appearance of moderate proteinuria (1.5 g/d), indomethacin was withdrawn and replaced by enalapril (0.5 µg·kg-1·d-1). After 3 mo of this treatment, porteinuria declined to the upper normal limit (150 mg/d).
At the last evaluation (age 13.2 y), the boy was in good clinical condition, and his height was 162 cm (+1.2 SD) (Fig. 2). Treatment consisted of KCl supplementation (1.5 mmol·kg-1·d-1) and enalapril (0.5 mg·kg-1·d-1). His serum potassium was 3.87 mM, serum magnesium, 0.73 mM, plasma HC03, 31.4 mM, and molar urinary calcium/creatinine, 0.26. The previous diagnosis of Bartter syndrome was questioned and Gitelman disease was considered.
SPECIAL METHODS
Hypertonic saline infusion test. The test were performed as described by Zerbe and Robertson(8). Patients were recumbent and fasted overnight from 2200 h; free access to water was allowed. Cannulas were inserted into the antecubital vein of each forearm, one for hypertonic saline infusion and the other for blood sampling. After taking two basal samples at 30-min intervals, infusion of 5% (855 mM) sodium chloride was begun at a rate of 0.05 mL·kg-1·min-1 for 3 h. Blood samples were drawn every 30 min into chilled, evacuated tubes containing a film of dried lithium-heparin for plasma osmolality measurement and EDTA for vasopressin determination. No fluid was ingested during the 3-h infusion period. Thirst was assessed by a visual analog scale(9). Subjects were given a sheet of paper with an uncalibrated 10-cm vertical line and asked to mark their estimation of thirst on the line between the extremes "no thirst" at the bottom and "very thirsty" at the top. For statistical analysis the thirst rating was defined as the distance in centimeters from zero ("no thirst"). Plasma osmolality was evaluated by freezing-point depression (Fiske Osmometer, Burlington, MA). The within-assay coefficient of variation was 0.7%. Vasopressin was assayed by Incstar kit (lower limit of detection for vasopressin was 0.2 pg/mL). Samples for vasopressin determinations were extracted using an octadecasilyl column according to the manufacturer's instructions. In our study, the extraction procedure had a recovery of about 82%, and the RIA had within-assay and between-assay coefficients of 5% and 6% variation, respectively.
Case 1. Plasma osmolality was 278 mosm/kg at baseline and rose progressively during hypertonic saline infusion to 318 mosm/kg at 180 min. This rise was accompanied by a small increase in vasopressin (maximum level, 0.5 pM) (Fig. 3). Urinary osmolality in a morning sample (517 mosm/kg) showed that the defect in vasopressin secretion was partial. Thirst increase (1.4 to 10) was vasopressin left-shifted compared with vasopressin secretion(8).
Case 2. At baseline, plasma osmolality was normal (279 mosm/kg) and rose during hypertonic saline infusion to 312 mosm/kg at 180 min. This rise was accompanied by an increase in vasopressin (from 0.4 pM at baseline to 6.2 pM at 180 min). However, the secretion was delayed compared with normal subjects (Fig. 3). Thirst rating was not performed because of the boy's neurologic problems.
Clearance studies. Solute reabsorption in Henle's loop and tubular segments beyond Henle's loop was evaluated in case 1 according to the free water clearance method with furosemide (1 mg/kg i.v.)(10). Free water clearance during the furosemide effect, indicating solute reabsorption after Henle's loop (expressed as percent reabsorption of solute load at each tubular site) was decreased at 52% (reference values, 58-96%). On the contrary, NaCl reabsorption occurring in Henle's loop (also expressed as percent reabsorption of solute load at each tubular site) was normal at 80% (reference values, 63-82%). These data were in accordance with an abnormal reabsorption of NaCl in the distal convoluted tubule(10).
Molecular genetic investigations. Molecular variants in the TSC, BSC (NKCC2 or SLC12A1), ROMK, and CLCNKB genes were investigated using SSCP(11). Twenty-seven specific primer pairs were used to amplify the entire coding region of the TSC gene including the intron-exon boundaries(2,3). Similarly, all exon sequences of the BSC and CLCNKB genes were screened using primers as reported by Simon et al.(12,13). Aberrant band patterns for the ROMK gene were studied using an overlapping set of primers designed by Simon et al.(14) to screen exons 4 and 5 spanning the complete coding region sequence of this channel gene.
PCR products of sizes ranging from 150 to 300 bp were separated at room temperature by electrophoresis at 35 W constant power for 4-6 h into a native 6% acrylamide gel (62.5 acrylamide: 1 bis-acrylamide) with 10% glycerol as described by Mastroianni et al.(15). Identified variants were reamplified with the same primer pairs, gel extracted, purified, and directly sequenced on both strands. The presence of a specific mobility shift was checked in 40 unrelated controls.
No mutations were found in the BSC, ROMK, and CLCNKB genes in either patient, even though the running conditions allowed detection of known mutations, loaded as positive controls. On the contrary, TSC exon analysis showed an aberrant band pattern in both patients.
A missense G186D, which was already known to be associated with Gitelman disease(15), was detected on the maternal allele in case 1. This amino acid replacement of a neutral glycine by a negatively charged aspartic acid is located in the second transmembrane domain, close to the first extracellular loop. A multiple protein alignment showed that G186 is conserved both in the TSC and the BSC genes of several species (Fig. 4). Case 2 is heterozygous for a novel mutation, a 1-bp deletion at position 2614 of the TSC gene, which results in a frameshift at amino acid 863, causing the loss of most of the intracellular carboxy terminus, including four potential phosphorylation sites. Both mutation carriers, carrier 1 and 2, were heterozygotes. The limited resolution power of the mutation detection technique SSCP could explain the missing mutation on the homologous chromosome in both cases. Nonetheless these two mutations are consistently pathogenetic and support the molecular diagnosis of Gitelman disease.
Local alignment of electroneutral cotransporter from different species: hTSC, rTSC, and flTSC denote human, rat, and flounder TSC, respectively; hBSC, rbBSC, and rBSC denote human, rabbit, and rat apical bumetanide-sensitive cotransporter; hBSC2, mBSC2, and sBSC2 denote human, mouse, and spiny dogfish basolateral bumetanide-sensitive cotransporter. The completely conserved glycine residue (G) at position 186 is mutated to aspartic acid (D) in case 1.
DISCUSSION
Two main findings characterized these two complex cases: the primary renal tubular origin of hypokalemia, later diagnosed as hereditary Gitelman disease, and the presence of an associated pituitary disorder.
Although the primary renal tubular origin of hypokalemia was identified at the onset of symptoms of the disease in both children, the molecular screening allowed us to better establish the diagnosis of Gitelman disease, especially considering differential diagnosis with Bartter syndrome. These two types of hereditary renal tubular disorders, associated with hypokalemia and metabolic alkalosis, may now be deemed entities with different phenotypes and genotypes(16). To date, three genes have been shown to be responsible for Bartter syndrome, i.e. BSC(12), ROMK(14), and CLCNKB(13) genes. The respective encoded proteins are ion transporters located in the ascending limb of Henle's loop, whereas the TSC protein is selectively expressed in the distal portion of the tubule and is currently considered the single cause of Gitelman disease.
The TSC gene mutations detected in our two patients, even though at the heterozygote state as discussed in the molecular genetics section, are likely to cause a loss of function of the Na-Cl cotransporter in the distal convoluted tubule. It is also well known that mutations can be missed by PCR-SSCP analysis or may be present in gene-regulating fragments such as promoter or enhancer segments, intron sequences, or 5′ and 3′ noncoding regions, which have not been screened for mutations(6). An abnormality in NaCl reabsorption in the distal tubule was also confirmed by a clearance study performed in patient 1. This defect results in NaCl loss, which leads to a secondary increase of NaCl delivery to distal nephron sites (mostly cortical collecting tubules), stimulating electrogenic sodium reabsorption accompanied by potassium and hydrogen ion hypersecretion at these sites(17). Hypokalemia and metabolic alkalosis ensue. Other factors in the pathogenesis of hypokalemia are increased potassium secretion stimulated by increased distal tubular flow(18) and secondary hyperaldosteronism. Prematurity, polyhydramnios, nephrocalcinosis, hypercalciuria, and neonatal onset of symptoms are features in the history of antenatal Bartter syndrome patients(19) but not Gitelman patients(4–6,16), including both of ours. Hypercalciuria may not be present in Bartter syndrome type III(13), but molecular evaluation was negative for the detection of mutations of this gene in our patients, excluding this third type of Bartter syndrome.
In Gitelman disease, hypomagnesemia and hypocalciuria are usually considered obligatory criteria for diagnosis(5,20), but were not demonstrated in our patients in most determinations. Colussi et al.(21,22). described three adult patients with Gitelman disease, one with normal calcium excretion values and two with normal serum magnesium, suggesting that hypocalciuria and hypomagnesemia may not be obligatory diagnostic markers of the disease. However, molecular diagnosis was not available in these cases.
Some additional comments on our case 2 are warranted. He presented peculiar features such as neuropsychological problems, signs of anticipated puberty, improved growth even after stopping GH treatment, and the appearance of proteinuria during indomethacin treatment. We have not found a syndrome that fits all the clinical and biochemical findings of this child. Puberty disorders have been associated with empty sella syndrome(23), and indomethacin treatment has been reported to induce catch-up growth with faster bone maturation in several hypokalemic patients with Bartter syndrome(24,25). Furthermore, indomethacin and other nonsteroidal drugs have been observed to induce proteinuria also in the nephrotic range(26,27), with amelioration after withdrawal of the drug. In case 2, proteinuria may have returned to the reference range because of indomethacin withdrawal and also enalapril treatment, which is used to correct hypokalemia in some patients with renal tubular hypokalemic alkalosis(28). The molecular study definitively established the diagnosis of Gitelman disease despite the presence of features not previously reported. The neurologic symptoms did not appear to be caused by Gitelman disease; they may have been a result of prenatal suffering, but we have not found a convincing answer to these problems.
The second striking finding in both cases was growth retardation caused by GH deficiency and altered vasopressin secretion associated with empty sella. Up to now, only one patient with Gitelman disease has been described as having growth retardation associated with GH deficiency(7), although two other children previously considered to have Bartter syndrome may be affected by Gitelman disease(29,30).
The exact incidence of growth retardation in Gitelman disease is not known but it appears to be much lower than in Bartter syndrome(5). Many factors that may contribute to growth retardation in Bartter syndrome such as prematurity, important salt loss in the antenatal-neonatal period, and marked polyuria are not present in Gitelman disease. In both disorders, hypokalemia and metabolic alkalosis may have some effect on growth but evidence in humans is lacking. At present, only experimental studies have demonstrated that potassium depletion plays a role in growth retardation, accompanied by reduced GH response to GH releasing factor and reduction of circulating IGF-I(31). Correcting the potassium deficiency in children with Bartter syndrome did not correct growth failure in early childhood(32). Other factors that may interfere with growth in these patients are abnormal glucose tolerance and hyperinsulinemia(32,33). However, the glucose tolerance test was normal in our two patients.
In both cases, growth failure appeared to be caused by GH deficiency associated with empty sella, an anatomic condition characterized by intrasellar arachnoid evaginations(34). The relative incidence of empty sella in adults and children is not known. It has been stated that two thirds of the individuals who present this particular anatomic variety are not symptomatic, nor do they present complications(35). In a recent study by Zucchini et al.(23) of a group of 43 subjects aged 4-27 y with empty sella, 20 had isolated GH deficiency, 17, multiple pituitary hormone deficiency, and six, pubertal disorders. The association between empty sella and diabetes insipidus or altered vasopressin secretion in children appears to be very rare(36,37). In case 1, a hypertonic saline infusion test demonstrated a partial defect in vasopressin secretion, and a stalk lesion may be hypothesized with lack of connection between the hypothalamus and pituitary gland. The renal osmolality of 517 mosm/kg may be caused by a leak of vasopressin from neurons of hypothalamic nuclei, as may be expected in patients with stalk lesion. Conversely, in case 2 the result of the hypertonic saline test was quantitatively normal but delayed. Abnormalities of vasopressin secretion have not previously been described in Gitelman disease. In a group of six adult patients who were reported to be affected by Bartter syndrome associated with hypocalciuria and hypomagnesemia (i.e. Gitelman disease), vasopressin values were normal(38).
Although empty sella syndrome is probably more frequent than previously suggested, the association between a rare hereditary tubular disorder such as Gitelman disease and a symptomatic pituitary disorder might be considered exceptional. Interestingly, the adult patient with Gitelman disease and normal urinary calcium excretion described by Colussi et al.(10) also had an empty sella at computed tomography despite normal endocrinologic findings. Thus, an association between empty sella and Gitelman disease should be considered in future studies.
Regarding the treatment of this variant of Gitelman disease, GH replacement treatment appeared effective in both our cases, as was desmopressin in case 1. It is unlikely that the growth response to GH is secondary to variations in urinary potassium wasting or serum potassium. In fact, hypokalemia persisted during GH treatment.
In conclusion, these two children may represent a new phenotype of Gitelman disease, and this entity may be considered a new complex hereditary renal tubular-pituitary syndrome. In patients with Gitelman disease and growth retardation, accurate molecular, endocrinologic, and instrumental tests appear crucial to study the pathogenesis of the disorder and to define the most rational therapeutic approach.
Abbreviations
- BSC:
-
bumetanide-sensitive Na-K-2Cl cotransporter
- CLCNKB:
-
chloride channel
- FE:
-
fractional excretion
- ROMK:
-
inwardly rectifying renal potassium channel
- SCCP:
-
single-strand conformation polymorphism analysis
- TSC:
-
thiazide-sensitive cotransporter
References
Gitelman HJ, Graham JB, Welt LG 1966 A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians 79: 221–235
Simon DB, Nelson-Williams C, Johnson Bia M, Ellison D, Karet FE, Morey Molina A, Vaara I, Iwata F, Cusher HM, Koolen M, Gianza FJ, Gitelman HJ, Lifton RP 1996 Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30
Mastroianni N, De Fusco M, Zollo M, Arrigo G, Zuffardi O, Bettinelli A, Ballabio A, Casari G 1996 Molecular cloning, expression pattern, and chromosomal localization of the human Na-Cl thiazide-sensitive cotransporter (SLC12A3). Genomics 35: 486–493
Rodriguez-Soriano J, Vallo A, Garcia-Fuentes M 1987 Hypomagnesemia of hereditary renal origin. Pediatr Nephrol 1: 465–472
Bettinelli A, Bianchetti MG, Girardin E, Caringella A, Cecconi M, Claris Appiani A, Pavanello R, Gastaldi R, Isimbaldi C, Lama G, Marchesoni C, Matteucci C, Patriarca C, Di Natale B, Setzu C, Vitucci P 1992 Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr 120: 38–43
Lemmink HH, Knoers N, Rarolyi L, van DijK H, Niaudet P, Antignac C, Guay-Woodford LM, Goodyer PR, Carel JC, Hermes A, Seyberth HW, Monnens LAH, van den Heudel LP 1998 Novel mutations in the thiazide-sensitive NaCl cotransporter gene in patients with Gitelman syndrome with predominant localization to the C-terminal domain. Kidney Int 54: 720–730
Ko CW, Koo JH 1997 Growth hormone (GH) deficiency may contribute to the occurrence of short stature in a child with Gitelman's syndrome. Am Soc Nephrol 7: 104A
Zerbe RL, Robertson GL 1981 A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med 305: 1539–1546
Baylis PH, Thompson CJ 1988 Osmoregulation of vasopressin secretion and thirst in health and disease. Clin Endocrinol 29: 549–576
Colussi G, Rombolà G, Verde G, Airaghi C, Loli P, Minetti L 1992 Distal nephron function in Bartter's syndrome: abnormal conductance to chloride in the cortical collecting tubule?. Am J Nephrol 12: 229–239
Orita M, Suzuki Y, Sekiya T, Hayasmi K 1989 Rapid and sensitive detection of point mutations and DNA polymorphism using the polymerase chain reaction. Genomics 5: 874–879
Simon D, Karet F, Hamdan J, Di Pietro A, Sanjad S, Lifton R 1996 Bartter's syndrome, hypokalemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188
Simon D, Bindra R, Mansfield T, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP 1997 Mutations in the chloride channel gene, CLCNKB, cause Bartter syndrome type III. Nat Genet 17: 171–178
Simon D, Karet F, Rodriguez-Soriano J, Hamdan J, Di Pietro A, Trachtman H, Sanjad SA, Lifton RP 1996 Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 12: 152–156
Mastroianni N, Bettinelli A, Bianchetti MG, Colussi G, De Fusco M, Sereni F, Ballabio A, Casari C 1996 Novel molecular variants of the Na-Cl cotransporter gene are responsible for Gitelman syndrome. Am J Hum Genet 59: 1019–1026
Bettinelli A, Vezzoli G, Colussi G, Bianchetti MG, Sereni F, Casari G 1998 Genotype-phenotype correlations in normotensive patients with primary renal tubular hypokalemic metabolic alkalosis. J Nephrol 11: 61–70
Gill JR Jr 1992 Disorders of renal transport of sodium, potassium, magnesium, and calcium. In: Edelman CH (ed) Pediatric Kidney Disease. Little, Brown and Company, Boston, pp 1873–1887
Wright FC, Giebisch G 1978 Renal potassium transport: contribution of individual nephron segments and populations. Am J Physiol 235:F515–F525
Seyberth HW, Rascher W, Schweer H, Kuhl PG, Mehls O, Scharer K 1985 Congenital hypokalemia with hypercalciuria in preterm infants: a hyperprostaglandinuric tubular syndrome different from Bartter syndrome. J Pediatr 107: 694–701
Quamme GA 1997 Renal magnesium handling: new insights in understanding old problems. Kidney Int 52: 1180–1195
Colussi G, Macaluso M, Brunati C, Minetti L 1994 Calcium metabolism and calciotropic hormone levels in Gitelman's syndrome. Miner Electrolyte Metab 20: 294–301
Colussi G, Rombolà G, Brunati C, De Ferrari ME 1997 Abnormal reabsorption of Na+/Cl- by the thiazide-inhibitable transporter of the distal convoluted tubule in Gitelman's syndrome. Am J Nephrol 17: 103–111
Zucchini S, Ambrosetto P, Carla G, Tani G, Franzoni E, Cacciari E 1995 Primary empty sella: differences and similarities between children and adults. Acta Paediatr 84: 1362–1365
Dillon MJ, Shah V, Mitchell MD 1979 Bartter's syndrome: 10 cases in childhood. Q J Med 191: 429–446
Floret D, David M, Roux A, Hage GN, Teyssier G 1979 Syndrome de Bartter: effects à long terme de l'indométacine sur la croissance. Nouv Presse Med 8: 17–21
Clive DM, Stoff JS 1984 Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1984: 310:56563-572
Lindsley CB, Warady BA 1990 Nonsteroidal antiinflammatory drugs. Clin Pediatr 29: 10–13
Hené RJ, Koomans HA, Dorhout Mees EJ, Stolpe AVD, Verhoef GEG, Boer P 1987 Correction of hypokalemia in Bartter's syndrome by enalapril. Am J Kidney Dis 9: 200–205
Ruvalcaba RHA, Martinez FE 1992 Case report: familial growth hormone deficiency associated with Bartter's syndrome. Am J Med Sci 303: 411–414
Boer LA, Zoppi G 1992 Bartter's syndrome with impairment of growth hormone secretion. [letter] Lancet 340: 860
Flyvbjerg A, Dorup I, Everts ME, Orskov H 1991 Evidence that pottasium deficiency induces growth retardation through reduced circulating levels of growth hormone and insulin-like growth factor I. Metabolism 40: 769–775
Simopoulos AP, Bartter FC 1972 Growth characteristics and factor influencing growth in Bartter's syndrome. J Pediatr 81: 56–65
Simopoulos AP 1979 Growth characteristics in patients with Bartter's syndrome. Nephron 23: 130–135
Kaufman B 1969 The "empty" sella turcica-a manifestation of the intrasellar subarachnoid space. Radiology 90: 931–941
Ferreri AJ, Garrido SA, Markarian MG, Yanez A 1992 Relationship between the development of diaphragma sellae and the morphology of the sella turcica and its content. Surg Radiol Anat 14: 233–239
Marano GD, Horton JA, Vazaquez AM 1981 Computed tomography in diabetes insipidus: posterior empty sella. Br J Radiol 54: 263–265
Hung W, Fitz CR 1992 The primary empty-sella syndrome and diabetes insipidus in a child. Acta Paediatr 81: 459–461
Stahl MMS, Vaara I, Hedner P, Ekmans R 1993 Vasoactive peptides in Bartter's syndrome. Eur J Clin Invest 23: 80–83
Acknowledgements
The authors thank the Associazione per il Bambino Nefropatico, Telethon Institute of Genetics and Medicine of Milan (TIGEM), and Ms Melissa Smith for the assistance in preparation of this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bettinelli, A., Rusconi, R., Ciarmatori, S. et al. Gitelman Disease Associated with Growth Hormone Deficiency, Disturbances in Vasopressin Secretion and Empty Sella: A New Hereditary Renal Tubular-Pituitary Syndrome?. Pediatr Res 46, 232–238 (1999). https://doi.org/10.1203/00006450-199908000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199908000-00017
This article is cited by
-
Classic Bartter syndrome complicated with profound growth hormone deficiency: a case report
Journal of Medical Case Reports (2013)
-
Longitudinal growth in chronic hypokalemic disorders
Pediatric Nephrology (2010)
-
A patient with Bartter syndrome accompanying severe growth hormone deficiency and focal segmental glomerulosclerosis
Clinical and Experimental Nephrology (2010)
-
Gitelman syndrome: when will it turn into Gitelman disease?
Pediatric Nephrology (2003)