Abstract
Factors affecting bone turnover in premature infants are not entirely clear but certainly are different from those influencing bones of adults and children.
To identify fetal and maternal factors that might influence bone turnover, we prospectively studied 50 infants (30 preterm and 20 full-term) born at Ain Shams University Obstetric Hospital in Cairo, Egypt. Maternal parity and medical history and infant's weight, gestational age, gender and anthropometrical measurements were recorded. Cord blood samples were collected and serum type I collagen C-terminal propeptide (PICP) was assessed as a marker for fetal bone formation. First morning urine samples were collected and pyridinoline cross-links of collagen (Pyd) were measured as an index for bone resorption. Serum PICP was higher in premature infants when compared with full-term infants (73.30 ± 15.1 versus 64.3 ± 14.7, p = 0.022) and was higher in male premature infants when compared with females (81.64 ± 9.06 versus 66.0 ± 15.7, p = 0.018). In a multiple regression model using PICP as the dependent variable and controlling for different infant and maternal conditions, PICP significantly correlated with infant gender (r = 8.26 ± 4.1, p = 0.05) maternal parity (r = −2.106 ± 0.99, p = 0.041) and diabetes (r = 22.488 ± 8.73, p = 0.041). Urine Pyd tended to increase in premature infants (612 ± 308 versus 434 ± 146, p = 0.057) and correlated significantly with gestational age (r = −63.93 ± 19.55, p = 0.002). Therefore, bone formation (PICP) is influenced by fetal age and gender, as well as maternal parity and diabetes. Bone resorption (Pyd) is mostly dependent on gestational age only. Further in-depth studies are needed to enrich management of this vulnerable population.
Similar content being viewed by others
Main
Throughout life, bone is constantly resorbed and new bone is formed. Both modeling and remodeling occur during skeletal growth. Bone modeling results in linear growth and remodeling allows expansion of bone circumference and mineral deposition. Five to twenty percent of the bone mass is actively remodeled by about two million bone remodeling units in the human skeleton at any given time (1,2). The rate of bone turnover in adults differs from children and neonates. In addition, factors affecting such process in adults do not necessarily influence children or neonates (3,4). Premature infants represent a unique vulnerable population, in which bone growth and mineral acquisition are critical. The relation of bone turnover with gestational age and birth weight has been well described (5). However, the impact of other factors such as parity, maternal diseases, and infant's sex on bone turnover in the premature infant are yet to be identified. In this study we used serum concentration of carboxy terminal propeptide of type I procollagen (PICP) in cord blood, as a marker of fetal bone formation (6–8), and pyridinoline (Pyd) excretion in urine as a biomarker of bone resorption (9,10).
PATIENTS AND METHODS
Patients.
Fifty appropriate-for-gestational-age infants were included in this study. Thirty preterm and twenty term infants were prospectively enrolled. Infants with history of perinatal asphyxia, maternal fever, rupture of amniotic membranes >18 h, congenital anomalies, liver diseases, and inherited metabolic or genetic disorders were excluded from the study. The study was approved by the institutional review board and parental consents were obtained. Maternal history including maternal age, parity and diabetes mellitus. Length of gestation was estimated using the date of last menstrual period, early antenatal ultrasound when available and the new Ballard scoring system (11). All anthropometrical measurements were done by the same doctor on admission and were plotted against gestational age (12). Complete blood counts, C- reactive protein, serum calcium phosphorus and alkaline phosphatase were measured. Samples were withdrawn without stasis and immediately analyzed. Carboxy-terminal propertide of type I procollagen in cord blood and urinary pyridinium cross-links were measured (13,14).
Carboxy terminal propeptide of type I procollagen (PICP) assay.
Blood was collected without anticoagulants and in such a way to avoid hemolysis. It was allowed to clot and was separated by centrifugation. Serum was frozen at −20°C and stored until use. For antibody incubation, 300 μL of 1× wash buffer was added to each well and strips manually inverted. This was repeated two more times for a total of three washes. The strips were vigorously blotted on paper towels after the last wash, and 100 μL of rabbit anti-CICP was added to each well (Metra Biosystems, Inc., San Diego, CA). Sample was then incubated for 45–50 min at room temperature (18–25°C). Enzyme conjugate was prepared in a similar manner within 2 h of use. OD at 405 nm was read after ensuring that no large bubbles were present in wells and the bottoms of the strips were clean. Quantitative software with a 4-parameter calibration curve was used to determine the final PICP concentration.
Urinary pyridinium cross-links assay.
Total pyridinoline (Pyd) in the first morning void was indexed by urinary creatinine (nmol/mmol creat). Collected urine samples were immediately frozen at −20°C until use. For substrate incubation, strips were manually inverted and 250 μL of 1× wash buffer was added to each well. This was repeated two more times for a total of three washes. The strips were vigorously blotted dry on paper towels after the last wash with cold (2–8°C) 1× wash buffer. Clean strips were inverted and allowed to drain on paper towels for 5–10 min to equilibrate to room temperature before adding substrate. 150 μL of working substrate solution was added to each well. They were then incubated for 60 min at room temperature (20–28°C). Subsequently, 100 μL of stop solution was added to each well. The OD at 405 nm was read after assuring that no large bubbles were present in the wells, and the bottoms of the strips were clean. Quantitative software with a 4-parameter calibration curve fitting equation was used to determine the pyrilinks assay results.
Statistical analysis.
Data were analyzed using The SAS System® Version 6.12 (15). Demographic data were analyzed using t test, Fisher's Exact test and Chi-Square test as appropriate. The Kruskal-Wallis Test was used to determine significant differences in biochemical markers between groups. All significant variables in the univariate analysis were entered into a multiple stepwise linear regression model to determine variables that had predictive values for the dependent determinant.
RESULTS
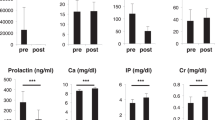
The demographic and anthropometric measurements of the study population are shown in Table 1. Serum PICP was higher in premature infants (n = 30) when compared with full-term infants (n = 20) (p = 0.022). Serum Pyd tended to be higher in premature infants but did not reach significance (p = 0.057). The lower serum calcium concentration in premature infants did not reach statistical significance when compared with term infants (Table 2).
PICP was significantly higher in male premature infants (81.64 ± 9.06 versus 66.0 ± 15.7; p = 0.018) when compared with females. There was no gender preference for any other bone turnover laboratory values (Table 3). PICP did not have any male-female gender bias in full-term infants (65.9 ± 15.7 versus 62.0 ± 15.1, respectively; p = 0.59).
In a multiple regression model using PICP as the dependent variable, and controlling for different infant and maternal conditions, PICP significantly correlated with infant gender, maternal parity and maternal diabetes. Other infant-related factors such as: gestational age, length, serum calcium, phosphorus and alkaline phosphatase were not correlated with PICP concentrations (Table 4, Fig. 1).
Applying the same regression model while using urinary Pyd as the dependent variable, significant correlation of Pyd was detected only with gestational age (Fig. 2). All other maternal and infant factors were not correlated with Pyd (Table 5).
DISCUSSION
The present study demonstrated premature infants to have a higher concentration of PICP in cord blood when compared with full-term infants. Cord blood PICP was significantly higher in male compared with female premature infants. Parity and maternal diabetes correlated with serum PICP. Urinary Pyd was influenced only by gestational age.
In this study, PICP concentration in cord blood samples was used as a marker of fetal bone formation. PICP is a cleavage of the carboxyterminal extension peptides of type I collagen, and each PICP molecule released in the blood corresponds with a collagen molecule deposited in the tissues (16). Since type I collagen makes up to 90% of the bone matrix, analysis of antigens related to collagen in the blood is expected to provide a reasonably specific estimate of bone formation (6). In addition, urinary excretion of their metabolites such as Pyd has been widely used as biochemical marker of bone resorption (9).
PICP in the cord blood of premature infants was higher when compared with full-term infants. This finding is in agreement with two previous studies (17,18). PICP was shown to peak at around 36 wk postmenstrual, and decrease thereafter throughout life (19). Interestingly, PICP concentration in cord blood is almost 50 times higher than its level in adults (16).
Urinary Pyd was also greater in preterm infants than in full-term infants, suggesting that premature infants have increased activity for bone resorption at birth (5). Although Pyd is not directly influenced by renal function in infancy, it is still indexed by urinary creatinine; a factor known to depend on protein intake and skeletal muscle mass, both of which are closely related to growth (20). When using such methodology, creatinine indexed-Pyd becomes more focused on the bone component of the skeletal changes. Pyd in the urine varied considerably, an observation that was previously explained by the uncertainty of the timing of urine collection (21). Of note, urinary excretion of Pyd is subject to a circadian rhythm, with higher rates of excretion at night. Early morning samples are thought however to be more accurate than the 24-h collections in reflecting bone resorption, thus all samples in this study were collected in the early mornings (22).
PICP concentration in cord blood was significantly higher in male versus female premature infants, but there was no PICP-related gender bias in term infants. Such an interesting observation follows a recognizable trend for testosterone hormone in utero. Testosterone levels are increased in male fetuses during the period of sexual differentiation and throughout the second trimester of pregnancy. At term, this difference is no longer observable. The gender-difference in serum PICP may reflect differences in the production of the collagen degradation markers by skeletal and nonskeletal cells in premature infants (23).
Increased maternal parity correlated negatively with PICP concentration, but did not influence urinary Pyd. The reason of such change in bone metabolism with a tendency toward less bone formation, without affecting bone resorption is not clear. Whether increased parity creates an added risk for the development of osteopenia in premature infants is not known. Such concept can only be validated with longitudinal prospective studies following premature infants born to mothers with various parities.
The relation of maternal diabetes on PICP measured in cord blood can be interesting. Diabetes is generally associated with delayed fetal maturity and increased weight; both of which are expected to positively influence PICP level. However, it was shown in a previous study that maternal diabetes does not influence PICP level when measured in amniotic fluid (24). Fetal expression of PICP in amniotic fluid depends primarily on fetal kidney functions to accurately reflect the actual plasma levels in the fetus. Furthermore, the class of severity of diabetes, the quality of sugar control and the estimated fetal weight gain subsequent to diabetes should all be accounted for before drawing conclusions on the impact of maternal diabetes on fetal PICP. Such future study will require a considerably large population size.
Gestational age was inversely correlated with urinary Pyd, indicating active resorption required for bone modeling and remodeling processes throughout gestation. Since the brain grows most actively during late gestation and early infancy, we repeated the regression analysis after adding head circumference to the model. Head circumference correlated significantly with urinary Pyd (r = 0.606, p = 0.005).
In conclusion, bone turnover in prematurely delivered infants is influenced by their gender, length of gestation as well as maternal parity and diabetes. More in depth studies of these factors can enrich our strategy of management in this vulnerable population.
Abbreviations
- PICP:
-
procollagen type-I carboxy-terminal propeptide
- Pyd:
-
pyridinoline
References
Ganong WF 2000 Hormonal control of calcium metabolism and physiology of bone. In: Ganong WF (ed) Review of Medical Physiology. Edn. Drawer L, California, pp 365–378
Bradbeer J 1992 Cell biology of bone remodeling. In: Edwards C, Lincoln D (eds) Recent Advances in Endocrinology and Metabolism. Churchill-Livingstone, Edinburgh, pp 95–115
Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137
Namgung R, Tsang R 2000 Factors affecting newborn bone mineral content: in utero: effects on newborn bone mineralization. Proc Nutr Soc 59: 55–63
Tsukahara H, Watanabe Y, Hirano S, Tsubokura H, Kimura K, Mayumi M 1999 Assessment of bone turnover in term and preterm newborns at birth: measurement of urinary collagen crosslink excretion. Early Hum Dev 53: 185–191
Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J 1993 Serum markers of type I collagen formation and degradation in metabolic bone disease : correlation with bone histomorphometry. J Bone Miner Res 8: 127–132
Crofton PM, Shrivastava A, Wade JC, Stephen R, Kelnar CJ, Lyon AJ, McIntosh N 1999 Bone and collagen markers in preterm infants: relationship with growth and bone mineral content over the first 10 weeks of life. Pediatr Res 46: 581–587
Risteli L, Risteli J 1993 Biochemical markers of bone metabolism. Ann Med 25: 385–393
Gfatter R, Braun F, Herkner K, Kohlross C, Hackl P 1997 Urinary excretion of pyridinium cross links and N-terminal cross linked peptide in preterm and term infants. Int J Clin Lab Res 27: 238–243
Marowska J, Kobylinska M, Lukaszkiewicz J, Talajko A, Rymkiewicz-Kluczynska B, Lorenc RS 1996 Pyridinium crosslinks of collagen as a marker of bone resorption rates in children and adolescents: normal values and clinical application. Bone 9: 669–677
Ballard JL, Khoury CJ, Wedig K, Wang L, Eilers-Walsman BL, Lipp R 1991 New Ballard score, expanded to include extremely premature infants. J Pediatr 119: 417–423
Usher RH, Mclean FH 1969 Intrauterine growth of live-born infants: Standards obtained from measurements of infants born between 25 and 44 weeks of gestation. J Pediatr 74: 901–910
Winterbottom N, Vernon S, Freeman K, Daniloff P, Garnero P, Seyedin S 1992 An immunoassay for the C-terminal propeptide of type I collagen. J Bone Min Res 7( Suppl 1): 254
Lieuw-A-Fa M, Sierra RI, Specker BL 1995 Carboxy-terminal propeptide of human type I collagen and pyridinium cross-links as markers of bone growth in infants 1 to 18 months of age. J Bone Miner Res 6: 849–853
SAS Institute Inc 1997 SAS/STAT Software: Changes and enhancements through release 6.12. Cary, NC 831–843
Risteli J, Risteli L 1999 Products of bone collagen metabolism. In: Seibel MJ, Robins SP, Bilezikian JP (eds) Dynamics of Bone and Cartilage Metabolism. Academic Press, San Diego, pp 275–287
Seibold-Weiger K, Wollmann HA, Ranke MB, Speer CP 2000 Plasma concentrations of the carboxyterminal propeptide of type I procollagen (PICP) in preterm neonates from birth to term. Pediatr Res 48: 104–108
Kajantie E, Dunkel L, Risteli J, Pohjavuori M, Andersson S 2001 Markers of type I and type III collagen turnover as indicators of growth velocity in very low birth weight infants. J Clin Endocrinol Metab 86: 4299–4306
Shiff Y, Eliakim A, Shainkin-Kestenbaum R, Arnon S, Lis M, Dolfin T 2001 Measurement of bone turnover markers in premature infants. J Pediatr Endocrinol Metab 14: 389–395
Fujimoto S, Kubo T, Tanaka H, Miura M, Seino Y 1995 Urinary pyridinoline and deoxypyridinoline in healthy children and in children with growth hormone deficiency. J Clin Endocrinol Metab 80: 1922–1928
Husain SM, Mughal Z, Williams G, Ward K, Smith CS, Dutton J, Fraser WD 1999 Urinary excretion of pyridinium crosslinks in healthy 4–10 year olds. Arch Dis Child 80: 370–373
Fraser WD 1998 The collagen crosslinks pyridinoline and deoxypyridinoline: a review of their biochemistry, measurement, and clinical application. J Clin Ligand Assay 21: 102–110
Zanze JC, Souberbielle C, Kindermans C, Rossignol C, Garabedian M 1997 Procollagen propeptide and pyridinium cross links as markers of type I collagen turnover: sex and age related changes in healthy children. J Clin Endocrinol Metab 82: 2971–2977
Harrast SD, Kalkwarf HJ 1998 Effects of gestational age, maternal diabetes, and intrauterine growth retardation on markers of fetal bone turnover in amniotic fluid. Calcif Tissue Int 62: 205–208
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aly, H., Moustafa, M., Amer, H. et al. Gestational Age, Sex and Maternal Parity Correlate with Bone Turnover in Premature Infants. Pediatr Res 57, 708–711 (2005). https://doi.org/10.1203/01.PDR.0000160591.70409.C8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000160591.70409.C8
This article is cited by
-
Relationship of caffeine regimen with osteopenia of prematurity in preterm neonates: a cohort retrospective study
BMC Pediatrics (2022)
-
Can serum periostin predict bronchopulmonary dysplasia in premature infants?
Pediatric Research (2022)
-
Could low birth weight and preterm birth be associated with significant burden of hip osteoarthritis? A systematic review
Arthritis Research & Therapy (2018)
-
Caffeine is a risk factor for osteopenia of prematurity in preterm infants: a cohort study
BMC Pediatrics (2018)