Abstract
The purpose of this study was to determine whether protein carbonyls and the lipid peroxidation product malondialdehyde (MDA) are elevated in plasma from very low birth weight (<1500 g) infants, whether they are affected by selenium supplementation, and whether they are associated with poor respiratory outcome or retinopathy. The study group comprised 173 infants enrolled in a randomized controlled trial of selenium supplementation. Plasma samples, collected before randomization, at 7 and 28 d after birth, and at 36 wk postmenstrual age, were analyzed for protein carbonyls and total MDA. Respiratory outcome was assessed as oxygen requirement at 28 d of age or 36 wk postmenstrual age and as number of days on oxygen. Protein carbonyl concentrations in very low birth weight infants were significantly higher than for adults but lower than for cord blood from term infants. Median values decreased significantly by 28 d, and there was no relationship with birth weight. MDA concentrations in very low birth weight infants overlapped the ranges for healthy adults and cord blood from term infants. They correlated positively with birth weight at 28 d but not at other times. Supplementation almost doubled plasma selenium concentrations, but carbonyls and MDA did not differ between the supplemented and unsupplemented groups. There were no significant differences in oxidant marker levels in infants who did or did not develop chronic lung disease or retinopathy. Protein carbonyls and MDA measurements in plasma do not show evidence of systemic oxidative stress in <1500-g infants and are not affected by selenium supplementation. Oxidative injury at sites such as the lung may be important in prematurity, but markers from such sites must be measured to relate to outcome and antioxidant supplementation.
Similar content being viewed by others
Main
Preterm infants who have immature lungs and require intensive care are exposed to more reactive oxidants than term infants. These can originate from the high concentrations of oxygen they require, xanthine oxidase activation during episodes of hypoxemia and reoxygenation (1), and neutrophil activation associated with inflammation (2–6). Free radical generation and oxidative injury are strongly implicated in CLD (also referred to as bronchopulmonary dysplasia), ROP, and IVH, the major complications of prematurity (1, 2, 7–9). These conditions have been termed the oxygen radical diseases of prematurity to emphasize their probable common pathogenesis (1).
Evidence for oxidative injury comes predominantly from measurements of biochemical markers of lipid peroxidation and protein oxidation. These studies have shown that oxidation products can be measured in plasma (10–14), urine (14–16), lung aspirates (17, 18), or breath (19–23) of premature infants. In a number of these studies, concentrations were higher in the lowest birth weight, high-risk infants (20–22), and were elevated either after hypoxic insult (12, 23–26) or in association with high Fio2 (15–18). However, direct evidence that oxidation plays a causative role in the diseases of prematurity is limited. Several groups have reported higher concentrations of oxidant markers in association with CLD (10, 13, 16, 26). Others found a positive association with mortality, ROP, and IVH, but not with CLD (23). However, it is not always clear whether these associations just reflect a higher disease incidence in the lowest birth weight infants who receive more oxygen. There is also, in some cases, uncertainty about assay specificity. For example, it is not clear whether the TBA assay we used previously to show an association with CLD that persisted after correction for birth weight (10) is a true measure of lipid peroxidation (see below). Further work is needed, therefore, before we can be certain whether oxidants contribute to the pathogenesis of these neonatal diseases.
If oxidants are important, then antioxidant supplementation has the potential to decrease oxidative injury and improve outcome. There is some evidence that vitamin E may decrease the severity of both IVH and ROP, although no clear benefit has been shown in the case of CLD (27, 28). There have been few other trials of antioxidant supplementation, and the question of whether biochemical oxidant markers are affected by antioxidants has not been investigated. Selenium, as a component of the glutathione peroxidases, is an important antioxidant (29). Very low levels have been found in premature infants (30–32), potentially putting them at risk of oxidative injury. We have recently carried out a multicenter controlled trial of selenium supplementation of preterm infants during the first month of life (33). Rather surprisingly, we found that supplementation gave no significant protection against CLD or ROP. We have now examined whether supplementation has an effect on oxidant markers.
The present study was undertaken to establish whether there is evidence for systemic lipid or protein oxidation in premature infants by measuring oxidation products in plasma. We have determined whether there are associations between concentrations of these markers and CLD or ROP and also whether they are affected by selenium supplementation. The study population was a subgroup of the infants involved in the selenium trial and included all infants cared for in two centers. Lipid peroxidation was assessed by an HPLC-based TBA assay that is considered to measure predominantly protein-bound MDA (34). As an index of protein oxidation, we measured protein carbonyls using a sensitive ELISA (35). Carbonyl groups are produced on proteins when they are oxidized, but can also represent covalently bound aldehyde products of lipid peroxidation (36, 37).
METHODS
Patient details.
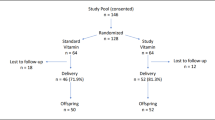
All infants with birth weight <1500 g and admitted within 48 h of birth to the Neonatal Intensive Care Unit at Christchurch Women's or Dunedin Hospital between 1994 and 1996 were eligible for study after their parents had signed informed consent. The study received approval from the Southern Regional Health Authority (Canterbury and Otago) Ethics Committees. There were 173 infants enrolled (136 in Christchurch and 37 in Dunedin), this being 78% of eligible infants. All infants were treated in accordance with standard protocols in the unit. Oxygenation was monitored by continuous pulse oximetry (Nelcor N100 & N200; initial target oxygen saturation was 87–95%) and frequent blood gas analysis. Arterial oxygen tension was maintained at 55–75 mm Hg. The hospital protocols called for antenatal glucocorticoids (three 8-mg doses of dexamethasone at 8-h intervals) to be administered to women in preterm labor at <34 wk gestation when possible. Postnatal steroids were administered at the neonatologist's discretion to infants of >7 d of age with Fio2 >50% and high mean airway pressure to aid weaning from positive-pressure ventilatory support. In these infants, dexamethasone was started at 0.5 mg/kg/d, then given at reducing doses during a 15- or 42-d course (mean dose, 0.08 mg/kg/d).
For infants unable to tolerate oral feeds, total parenteral nutrition was commenced on d 2 to 3 with 1 g/kg/d Vaminolact (Kabi Pharmacia, Auckland, New Zealand) and 10–15% dextrose. Intralipid 20% (Kabi Pharmacia) was commenced on d 3 to 4 at 0.5 g/kg/d. Both were increased gradually as tolerated to a maximum of 3 g/kg/d. Supplementary trace elements (Ped-El, Kabi Pharmacia, Stockholm, Sweden; 4 mL/kg/d) and vitamins (MVI-Paediatric, Rhone-Polenc Rorer, Lower Hutt, New Zealand; 2 mL/kg/d) were routinely added to total parenteral nutrition. In a double blinded fashion, infants were randomized to receive either selenium or a placebo, as described in detail elsewhere (33). Parenteral supplementation was with 7 μg/kg/d sodium selenate, and oral supplementation was with 5 μg/kg/d sodium selenite. Supplementation was continued until 36 wk PMA or hospital discharge if earlier.
Biochemical analyses.
Blood samples (0.6 mL) were collected into heparin at the time of routine sampling, and often from an indwelling arterial or venous catheter, before randomization for selenium supplementation (mean, 2.6 d), at 7 and 28 d after birth, and at discharge or 36 wk PMA. Samples were placed immediately in a dark refrigerator, and plasma was separated within 12 h and stored at −80°C until analysis. This procedure is acceptable for MDA analysis (38), and we have also found protein carbonyls to be stable for years at −80°C and at least a day at room temperature.
Plasma samples were heated with TBA, and the TBA-MDA adduct was analyzed by HPLC with fluorescence detection (39). Protein carbonyls were determined by an ELISA involving reaction of the protein with dinitrophenylhydrazine and detection with an anti-dinitrophenylhydrazine antibody (35). Total protein concentrations were measured for reference using the Bio-Rad assay (Bio-Rad Laboratories, Richmond, CA, U.S.A.). Plasma selenium was analyzed using an atomic absorption spectrometer (Varian Spectra AA40, Varian Industries, Sunnyvale, CA, U.S.A.) with Zeeman background correction. Glutathione peroxidase was measured with t-butyl hydroperoxide as substrate on a centrifugal analyzer (40). To assess relationships between different TBA assays, a selection of 2- and 7-d plasmas were also analyzed by the HPLC method of Wade and van Rij (41), in which lipids are extracted and heated with TBA in the presence of iron to generate the MDA-TBA complex. Plasma fatty acid profiles were also determined by gas chromatography after methylation.
Outcome measures.
Primary outcome measures, as for the randomized controlled trial of selenium supplementation (33), were defined as oxygen dependency at 28 d of age, and total number of days of oxygen dependency. Secondary outcome measures included death, death from d 7 to d 28 or oxygen dependency at 28 d of age, oxygen dependency at 36 wk PMA, death from d 7 to 36 wk PMA or oxygen dependency at 36 wk PMA, and ROP. ROP was assessed according to our routine screening protocol (42), with infants having an initial examination at 6 wk of age by an ophthalmologist skilled in indirect ophthalmoscopy. Retinopathic changes were recorded using the International Classification of Retinopathy of Prematurity (43).
Statistical analysis.
Statistical analyses were performed using SigmaStat (Jandel Scientific, San Rafael, CA, U.S.A.). Differences between groups were assessed using the Mann-Whitney or Kruskal-Wallis test. Relationships between variables were assessed using linear regression when the data were normally distributed, or using Spearman's rank order correlation.
RESULTS
MDA and protein carbonyls in infant plasma.
The characteristics of the <1500-g (VLBW) infants from whom plasma was collected are shown in Table 1. We considered them in two groups: those with birth weight <1000 g and those with birth weight 1000–1500 g (subsequently referred to as ≥1000 g). Concentrations of the lipid peroxidation product, MDA, in plasma in the period after birth overlapped the ranges for healthy, nonsmoking adults and cord blood from term infants (Fig. 1A). Median values for ≥1000-g infants were significantly lower (p < 0.05) than for cord blood from term infants but no different from the adult mean. They did not change significantly during the period of the study. The median for <1000-g infants was no different from that for ≥1000-g infants at 2 or 7 d but increased 1.4-fold (p < 0.05) at 28 d. This is reflected by negative correlations between MDA concentration and birth weight and gestational age at 28 d, but not at earlier times (Table 2).
Plasma MDA (A) and protein carbonyl (B) concentrations at different times for 1000- to 1499-g (clear boxes) and <1000-g (hatched boxes) infants. Box plots show medians and interquartile ranges, with error bars marking the 10th and 90th percentiles. Sample numbers (n) in each group are given. Results for healthy adults and cord blood samples from term infants are also shown.
Protein carbonyl concentrations in cord plasma from term infants were substantially higher than in adults (Fig. 1B). Values for both the <1000-g and ≥1000-g groups, at all times, varied over a wider range and were significantly higher than for healthy adults (p < 0.01) but were less than that for cord blood from term infants (p < 0.05). There were no significant differences between the two groups and no correlation with gestational age or birth weight. There was a significant decrease of approximately 30% between 7 and 28 d (p < 0.05) in both groups.
Effects of selenium.
There were no significant differences in birth weight or gestational age between the selenium-supplemented and -unsupplemented infant populations. Of the supplemented infants, 76% received antenatal steroids and 27% postnatal steroids compared with 87% and 32%, respectively, for the unsupplemented infants. Supplementation resulted in a significant increase in plasma selenium, to almost double the pretreatment concentration (Table 3). Without supplementation, there was a decrease as a function of time. Most of the increase caused by selenium had occurred by 1 wk of age, which is within an average of 4–5 d of commencing supplementation. Supplementation prevented the decline after birth in activity of the selenium-containing enzyme, glutathione peroxidase. This response was less pronounced than for selenium concentration, but it was also rapid, with differences between the two groups already significant at 1 wk (Table 3). The correlation between the two factors was weak for the prerandomization samples (correlation coefficient, 0.17;p = 0.04) and increased to between 0.33 and 0.49 (p < 0.001) at the other times. There were no significant differences in initial selenium and glutathione peroxidase between the <1000-g and ≥1000-g populations, and both responded similarly to supplementation.
Plasma protein carbonyl and MDA concentrations did not differ significantly between the supplemented and unsupplemented groups at any time, either when data for the whole population were analyzed (Table 4) or when the <1000-g and ≥1000-g groups were examined separately (not shown). Carbonyl concentrations did not correlate with selenium or glutathione peroxidase activity at any time. Apart from a weak negative correlation with glutathione peroxidase at 36 wk (correlation coefficient, −0.23;p = 0.01), MDA was not correlated with selenium or glutathione peroxidase at any time.
Relationship between oxidant markers and outcome.
As there was no significant effect of selenium supplementation on plasma concentrations of the oxidant markers or, as shown elsewhere, on respiratory outcome or development of retinopathy (33), relationships between oxidant markers and outcome were assessed for the whole population, regardless of selenium supplementation. MDA concentrations at 28 d, but not at other times, were significantly higher in those infants who required oxygen at 28 d or 36 wk, and there was a similar trend for ROP (Table 5). These differences were not seen when only <1000-g infants were considered. There was also a positive correlation between MDA measured at 28 d and days of oxygen requirement, but this did not remain significant after correcting for gestational age or birth weight by multiple regression analysis. Protein carbonyls at any time were no different for infants who did and did not acquire CLD or retinopathy, and did not correlate with days on oxygen. Including the infants who died with those who had CLD or ROP did not change these conclusions. At 28 d, but not other times, carbonyls were lower in those infants who received antenatal steroids (median, 0.114;n = 90) than in those who did not (median, 0.146;n = 13;p = 0.05). Antenatal or postnatal steroid administration did not affect other variables. MDA concentrations were 14% lower in samples (n = 139) collected when the infants were receiving any supplemental oxygen compared with those breathing air (n = 122;p < 0.05). Protein carbonyls were not related to Fio2 at the time of sampling.
Comparison of different TBA assays as indices of lipidperoxidation.
In the present study, we did not see higher MDA values at 7 d in infants who acquired CLD, as we observed previously (10). We investigated whether this could be related to the different assays used. Both assays use HPLC to separate the TBA adduct of the lipid peroxidation product MDA, and are assumed to be an index of lipid peroxidation. However, the procedure we used previously (10, 11) measures the MDA-TBA generated when lipid extracts are heated with iron as described by Wade and van Rij (41) and Wade et al. (44), whereas the current assay measures predominantly protein-bound MDA (34, 39). To compare the two assays, we analyzed 32 infant plasma samples. MDA-TBA concentrations ranged from 0.9 to 2.8 μM in the current assay compared with 1.0 to 4.1 μM in the previous assay. Correlation between the two sets of results was poor (r2 = 0.07). Consistent with earlier findings, concentrations measured with the assay of Wade and van Rij (41) and Wade et al. (44) were higher at 7 d than at 2 d. To assess whether this assay reflects the polyunsaturated fatty acid content of the sample and therefore a susceptibility to peroxidation when heated with iron, we measured arachidonic acid (the major source of MDA) and linoleic acid concentrations in the plasmas. Their concentrations ranged from 0.30 to 0.75 mM and 0.53 to 2.67 mM, respectively. Neither arachidonic acid nor the sum of the two correlated positively with MDA-TBA measurements (slopes, −1.62 μM/mM, r2 = 0.27 and −0.16 μM/mM, r2 = 0.06, respectively). The assay, therefore, is not a measure of the amount of substrate capable of undergoing peroxidation.
DISCUSSION
It is widely accepted that free radical generation and oxidative injury contribute to the diseases of prematurity (2, 9, 21), although few direct associations between oxidation and disease severity have been established. If there were generalized oxidative stress in VLBW infants, then concentrations of oxidant markers in plasma would be expected to be higher than in healthy populations. Furthermore, if oxidation plays a causative role in these diseases, higher concentrations would be expected in the sicker infants. We have examined plasma from a large group of <1500-g infants. Concentrations of MDA, measured as an index of lipid peroxidation, overlapped the range for healthy adults and were on average lower than for cord blood from term infants. Protein carbonyl concentrations, although higher and wider ranging than for adults, were also on average less than for cord plasma from term infants. These data provide no strong evidence for generalized oxidative stress in VLBW infants.
Several groups have measured elevated MDA concentrations in cord blood and suggested that this may reflect hypoxia and the stresses associated with birth (23, 25, 26). Others have observed an increase in cord plasma MDA with gestational age (12). Whether the higher cord carbonyl concentrations reflect stresses at birth or a difference between term and VLBW infants needs further investigation.
There were also no detectable associations between MDA or carbonyls and either CLD or ROP. In contrast to our previous studies (10, 11), we saw no positive association between MDA at 1 wk and poor respiratory outcome. We believe the explanation for this difference lies with the assay procedures. The assay used in this study is thought to measure predominantly protein-bound MDA as well as any peroxides present in plasma as a result of lipid peroxidation. It is unclear what the assay used previously measures. The lack of correlation with the other method, as well as the low peroxide levels in plasma (45), make it unlikely that it measures lipid peroxides as initially proposed (41), and we have now established that it does not reflect the amount of lipid in the samples that is capable of undergoing peroxidation. So, although the association with poor outcome is interesting, we cannot say with certainty that it involves lipid peroxidation.
The reason why our plasma indices provided little evidence of oxidative injury in VLBW infants could be that they are not specific enough to detect systemic oxidative stress. Alternatively, oxidation could be confined to particular sites such as the lung or retina and not be detectable in plasma. Lack of specificity is probably unlikely. Protein carbonyl measurements are frequently used to assess protein oxidation. Although carbonyls can arise through processes other than oxidative ones, elevations have been detected in other instances of oxidative stress (46, 47), with particularly high concentrations measured by ELISA in plasma and lung fluid from critically ill patients with adult respiratory distress syndrome (48). Although the TBA assay has been criticized as a measure of lipid peroxidation (34, 49), the version of the HPLC method we used is accepted as the least open to interference.
It seems more likely that oxidative stress is localized, and elevations of protein and lipid markers become lost when diluted in plasma. This is suggested by our findings of considerably higher protein carbonyl and MDA concentrations in tracheal aspirates from a similar premature infant population (50). Others have also obtained evidence for enhanced lung carbonyl formation (17, 18). Unfortunately, large population studies involving measurements on lung aspirates are difficult. Newer, more sensitive tests may be more discriminatory. Higher plasma concentrations of 8-isoprostane, a specific end product of lipid peroxidation (51), have been measured in premature infants than in adults (52), and elevated plasma levels of nitric oxide-derived nitrotyrosine have been found in infants who had CLD (13). These markers are potentially useful probes for further investigation.
As the selenium status of premature infants is low at birth and declines further without nutritional supplementation (30–32), we reasoned that this could compromise their antioxidant capacity and allow more protein and lipid oxidation. As expected for a New Zealand population, we found that plasma selenium concentrations at birth were low by world standards. These almost doubled within less than a week of starting supplementation. A significant difference in glutathione peroxidase concentration between the supplemented and unsupplemented infants was also evident, even though this requires new enzyme synthesis. Any difference in antioxidant protection between the two groups would therefore be expected to be evident from that time. As we have reported elsewhere, selenium supplementation did not result in significant clinical benefit (33). Given that we have now also shown that plasma markers showed little evidence of oxidative stress, it is perhaps not surprising that supplementation resulted in no significant differences in plasma carbonyls or MDA concentrations.
Failure to show benefit from selenium supplementation may have been because the amount given was insufficient, or because the low selenium status of the infants was already adequate. Alternatively, if oxidative injury is an early critical event, selenium supplementation may have been commenced too late to be beneficial. This possibility is perhaps supported by our finding that lower selenium concentrations in mothers, or in infants before randomization, were associated with increased risk of adverse outcome (33). In addition, there is increasing evidence that the inflammatory changes that are likely precursors of neonatal CLD occur within a few hours of birth (53) or even before delivery in some infants (54).
In conclusion, we found that protein carbonyls and MDA concentrations in plasma of VLBW infants were not indicative of systemic oxidative stress and did not correlate with respiratory outcome or ROP. They were also not affected by selenium supplementation. These findings do not necessarily discount the involvement of oxygen radicals in the diseases of prematurity, but suggest it is more likely to be a localized and subtle phenomenon.
Abbreviations
- CLD:
-
chronic lung disease
- IVH:
-
intraventricular hemorrhage
- MDA:
-
malondialdehyde
- PMA:
-
postmenstrual age
- ROP:
-
retinopathy of prematurity
- TBA:
-
thiobarbituric acid
- VLBW:
-
very low birth weight (<1500 g)
- Fio2:
-
inspired fraction of oxygen
- HPLC:
-
high-performance liquid chromatography
References
Saugstad OD 1996 Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta Paediatr 85: 1–4
Zimmerman JJ 1995 Bronchoalveolar inflammatory pathophysiology of bronchopulmonary dysplasia. Clin Perinatol 22: 429–457
Merritt TA, Stuard ID, Puccia J, Wood B, Edwards DK, Finkelstein J, Shirpiro DL 1981 Newborn tracheal aspirate cytology: classification during respiratory distress syndrome and bronchopulmonary dysplasia. J Pediatr 98: 949–956
Groneck P, Gotze-Speer B, Opperman M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high risk preterm neonates. Pediatrics 93: 712–718
Groneck P, Speer CP 1995 Inflammatory mediators and bronchopulmonary dysplasia. Arch Dis Child 73: F1–F3
Contreras M, Hariharan N, Lewandoski JR, Ciesielski W, Koscik R, Zimmerman JJ 1996 Bronchoalveolar oxyradical inflammatory elements herald bronchopulmonary dysplasia. Crit Care Med 24: 29–37
Saugstad OD 1997 Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the pathogenesis of BPD?. Acta Paediatr 86: 1277–1282
Rao RA 1996 Oxygen free radicals and retinopathy of prematurity. Brit J Opthalmol 80: 387
Kelly FJ 1993 Free radical disorders of preterm infants. Br Med Bull 49: 668–678
Inder TE, Darlow BA, Sluis KB, Winterbourn CC, Graham P, Sanderson K, Taylor BJ 1996 The correlation of elevated levels of an index of lipid peroxidation (MDA-TBA) with adverse outcome in the very low birth weight infant. Acta Paediatr 85: 1116–1122
Inder TE, Graham P, Sanderson KJ, Taylor BJ 1994 Lipid peroxidation as a measure of oxygen free radical damage in the very low birth weight infant. Arch Dis Child 70: F107–F111
Schmidt H, Grune T, Mueller R, Siems WG, Wauer RR 1996 Increased levels of lipid peroxidation products malondialdehyde and 4-hydroxynonenal after perinatal hypoxia. Pediatr Res 40: 15–20
Banks BA, Ischiropoulos H, McClelland M, Ballard PL, Ballard RA 1998 Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics 101: 870–874
Drury JA, Nycyk JA, Cooke RW 1997 Comparison of urinary and plasma malondialdehyde in preterm infants. Clin Chim Acta 263: 177–185
Lubec G, Widnes JA, Hayde M, Menzel D, Pollak A 1997 Hydroxyl radical generation in oxygen-treated infants. Pediatrics 100: 700–704
Schlenzig JS, Bervoets K, von Loewenich V, Boehles H 1993 Urinary malondialdehyde concentration in preterm neonates: is there a relationship to disease entities of neonatal intensive care?. Acta Paediatr 82: 202–205
Gladstone IM Jr, Levine RL 1994 Oxidation of proteins in neonatal lungs. Pediatrics 93: 764–768
Varsila E, Pesonen E, Andersson A 1995 Early protein oxidation in the neonatal lung is related to development of chronic lung disease. Acta Paediatr 84: 1296–1299
Wispe JR, Bell EF, Roberts RJ 1985 Assessment of lipid peroxidation in newborn infants and rabbits by measurements of expired ethane and pentane: influence of parenteral lipid infusion. Pediatr Res 19: 374–379
Pitkänen OM, Hallman M, Andersson SM 1990 Correlation of free oxygen radical-induced lipid peroxidation with outcome in very low birth weight infants. J Pediatr 116: 760–764
Varsila E, Hallman M, Andersson S 1994 Free-radical-induced lipid peroxidation during the early neonatal period. Acta Paediatr 83: 692–695
Varsila E, Pitkanen O, Hallman M, Andersson S 1994 Immaturity-dependent free radical activity in premature infants. Pediatr Res 36: 55–59
Nycyk JA, Drury JA, Cooke RW 1998 Breath pentane as a marker for lipid peroxidation and adverse outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed 79: F67–F69
Smith CV, Hansen TN, Martin NE, McMicken HW, Elliott SJ 1993 Oxidant stress responses in premature infants during exposure to hyperoxia. Pediatr Res 34: 360–365
Rogers MS, Mongelli JM, Tsang KH, Wang CC, Law KP 1998 Lipid peroxidation in cord blood at birth: the effect of labour. Br J Obstet Gynaecol 105: 739–744
Buonocore G, Zani S, Perrone S, Caciotti B, Bracci R 1998 Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic Biol Med 25: 766–770
Raju TNK, Langenberg P, Bhutani V, Quin GE 1997 Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr 131: 844–850
Ehrenkranz RA, Mercurio MR 1992 Bronchopulmonary dysplasia. In: Sinclair JC, Bracken MB (eds) Effective Care of the Newborn Infant. Oxford University Press, Oxford, pp 399–424
Levander OA 1987 A global view of human selenium nutrition. Ann Rev Nutr 7: 227–250
Lockitch G, Jacobson B, Quigley G, Dison P, Pendray M 1988 Selenium deficiency in low birth weight neonates: an unrecognized problem. J Pediatr 114: 865–870
Sluis KB, Darlow BA, George PM, Mogridge N, Dolamore BA, Winterbourn CC 1992 Selenium and glutathione peroxidase levels in premature infants in a low selenium community (Christchurch, New Zealand). Pediatr Res 32: 189–194
Darlow BA, Inder TE, Graham PJ, Sluis KB, Malpas TJ, Taylor BJ, Winterbourn CC 1995 Selenium status in the very low birth weight infant: relationship to maternal status, infant nutrition and respiratory outcome. Pediatrics 96: 314–319
Darlow BA, Winterbourn CC, Inder TE, Graham PJ, Harding JE, Weston PJ, Austin NC, Elder DE, Mogridge N, Buss IH, Sluis KB 2000 The effect of selenium supplementation on outcome in very low birth weight infants: a randomized controlled trial. J Pediatr 136: 473–480
Janero DR 1990 Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9: 515–540
Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC 1997 Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med 23: 361–366
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn B-W, Shalteil S, Stadtman ER 1990 Determination of carbonyl content of oxidatively modified proteins. Methods Enzymol 186: 464–478
Reznick AZ, Cross CE, Hu ML, Suzuki YJ, Khwaja S, Safadi A, Motchnik PA, Packer L, Halliwell B 1992 Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem J 286: 607–611
Wong SHY, Knight JA, Hopfer SM, Zaharia O, Leach CN Jr, Sunderman FW Jr 1987 Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33: 214–220
Young IS, Trimble ER 1991 Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Ann Clin Biochem 28: 504–508
Jaskot RH, Charlet EG, Grose EC, Grady MA, Roycroft JH 1983 An automated analysis of glutathione peroxidase, S-transferase, and reductase activity in animal tissue. J Anal Toxicol 7: 86–88
Wade CR, van Rij AM 1988 Plasma thiobarbituric acid reactivity: reaction conditions and the role of iron, antioxidants and lipid peroxy radicals on the quantitation of plasma lipid peroxides. Life Sci 43: 1085–1093
Darlow BA, Clemett RS 1990 Retinopathy of prematurity; screening and optimal usage of the ophthalmologists time. Aust N Z J Opthalmol 18: 41–46
Committee for the Classification of Retinopathy of Prematurity. 1984 An international classification of retinopathy of prematurity. Pediatrics 74: 127–133
Wade CR, Jackson PG, van Rij AM 1985 Quantitation of malondialdehyde (MDA) in plasma, by ion-pairing reverse phase high performance liquid chromatography. Biochem Med 33: 291–296
Bowry VW, Stanley KK, Stocker R 1992 High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A 89: 10316–10320
Smith MA, Perry G, Sayre LM, Anderson VE, Beal MF, Kowalll N 1996 Oxidative damage in Alzheimer's. Nature 382: 120–121
Ciolino HP, Levine RL 1997 Modification of proteins in endothelial cell death during oxidative stress. Free Radic Biol Med 22: 1277–1282
Winterbourn CC, Buss IH, Chan TP, Plank LD, Clark MA, Windsor JA 2000 Protein carbonyl measurements show evidence of early oxidative stress in critically ill patients. Crit Care Med 28: 143–149
Draper HH, Hadley M 1990 Malondialdehyde determination as an index of lipid peroxidation. Methods Enzymol 186: 421–431
Buss IH, Darlow BA, Winterbourn CC 2000 Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr Res 47: 640–645
Morrow JD, Roberts LJ 1997 The isoprostanes: current knowledge and directions for future research. Biochem Pharmacol 51: 1–9
Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, Roberts LJ, Frei B 1997 Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem 272: 15656–15660
Murch SH, Costeloe K, Klein NJ, MacDonald TT 1996 Early production of macrophage inflammatory protein-1 alpha occurs in respiratory distress syndrome and is associated with poor outcome. Pediatr Res 40: 490–497
Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI 1997 Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1b, and interleukin-8) and the risk of development of bronchopulmonary dysplasia. Am J Obstet Gynecol 177: 825–830
Acknowledgements
The authors thank Dr. Nicola Austin for coordinating the Dunedin arm of the study, Karl Sluis for assistance with sample handling, Sue Grant for selenium analyses, and Dr. Wayne Sutherland and Vicky Phillips for carrying out the fatty acid analyses. We also thank the staff of the Neonatal Units of Christchurch Women's and Dunedin Hospitals for assistance with sample collection.
Author information
Authors and Affiliations
Additional information
Supported by the Health Research Council of New Zealand and Lotteries Health Research.
Rights and permissions
About this article
Cite this article
Winterbourn, C., Chan, T., Buss, I. et al. Protein Carbonyls and Lipid Peroxidation Products as Oxidation Markers in Preterm Infant Plasma: Associations with Chronic Lung Disease and Retinopathy and Effects of Selenium Supplementation. Pediatr Res 48, 84–90 (2000). https://doi.org/10.1203/00006450-200007000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200007000-00015