Abstract
Heart rate (HR) has been widely studied as a measure of an individual's response to painful stimuli. It remains unclear whether changes in mean HR or the variability of HR are specifically related to the noxious stimulus (i.e. pain). Neither is it well understood how such changes reflect underlying neurologic control mechanisms that produce these responses, or how these mechanisms change during the first year of life. To study the changes in cardiac autonomic modulation that occur with acute pain and with age during early infancy, the relationship between respiratory activity and short-term variations of HR (i.e. respiratory sinus arrhythmia) was quantified in a longitudinal study of term born healthy infants who underwent a finger lance blood collection at 4 months of age (n = 24) and again at 8 months of age (n = 20). Quantitative respiratory activity and HR were obtained during baseline, lance, and recovery periods. Time and frequency domain analyses from 2.2-min epochs of data yielded mean values, spectral measures of low (0.04-0.15 Hz) and high (0.15-0.80 Hz) frequency power (LF and HF), and the LF/HF ratio. To determine sympathetic and parasympathetic cardiac activity, the transfer relation between respiration and HR was used.
At both 4 and 8 months, mean HR increased significantly with the noxious event (p > 0.01). There were age-related differences in the pattern of LF, HF, and LF/HF ratio changes. Although these parameters all decreased (p > 0.01) at 4 months, LF and LF/HF increased at 8 months and at 8 months HF remained stable in response to the noxious stimulus. Transfer gain changes with the lance demonstrated a change from predominant vagal baseline to a sympathetic condition at both ages. The primary finding of this study is that a response to an acute noxious stimulus appears to produce an increase in respiratory-related sympathetic HR control and a significant decrease in respiratory-related parasympathetic control at both 4 and 8 months. Furthermore, with increasing age, the sympathetic and parasympathetic changes appear to be less intense, but more sustained.
Similar content being viewed by others
Main
In the search for a better understanding of the infant pain experience, markers of autonomic arousal have been widely studied as a window into the neurologic substrate that results in the response to the noxious event(1–3). Randich and Maixner(4) demonstrated that systems controlling cardiovascular function are closely coupled to systems modulating perception of pain. It is not surprising therefore that many changes in cardiovascular function, regional blood flow, respiratory patterns, and oxygen saturation have been studied as measures of a response to a noxious event among infants.
HR has been used widely as a measure of an individual's response to painful stimuli. An increase in infant mean HR(5–7) and variability of mean HR(8) has been found after heel lances. Decreased parasympathetic or vagal tone as quantified from HR variability that occurs with respiration has been reported after circumcision(1). It appears that such responses have a developmental character that may reflect maturation of an autonomic response to stress(9–12). Mean HR declines during the first year of life(13), suggesting a possible increase in parasympathetic or decrease in sympathetic cardiac modulation in addition to other nonautonomic influences. Although a functional autonomic system is present in preterm(14) and term infants(15), there may be incomplete sympathetic nervous system development and a relative maturity of the parasympathetic system at birth(16). Recent work by Johnston et al.(17) indicates that mean HR in response to blood collection lance also appears to decline with infant maturity, indirectly suggesting a developmental character of cardiac autonomic pain response. However, it remains unclear how these changes in mean HR or HR variability are specifically related to the noxious stimulus (i.e. pain); neither is it well understood how these changes reflect underlying neurologic control mechanisms that result in these responses, or how these mechanisms change during the first year of life.
Analysis of short-term variations in HR may help us understand the neurologic and age-related responses to noxious events during infancy. Variability in HR is mediated primarily by changing levels of parasympathetic and sympathetic outflow from the CNS to the sinoatrial node at the heart. The HR power spectrum typically contains a peak at the respiratory frequency representing RSA and peaks at lower frequencies between 0.01 and 0.15 Hz. Studies using selective pharmacologic blockade of the cardiac sympathetic and parasympathetic receptors have shown that fluctuations in HR greater than 0.15 Hz are mediated exclusively by changes in parasympathetic activity, whereas lower frequency changes are mediated jointly by changes in parasympathetic and sympathetic efferent activity(18). Thus, with spectral analysis, short-term HR variability can be used to quantify changing levels of cardiac autonomic modulation.
Parasympathetic influences affecting HR via the vagus nerve are easier to identify, therefore, these autonomic influences have been studied more than the diffuse sympathetic influences. Therefore, most of the work investigating autonomic modulation of HR has focused on variations in HR that occur with respiration (i.e. 0.15-1.0 Hz, RSA). Some investigators have suggested that infant HR variability associated with RSA may be a measure of individual differences in stress response, infant development, and the functional status of the CNS and autonomic control mechanisms(19,1). A so-called vagal tone index, however, may not quantify the entire spectrum of autonomic arousal, and this limitation has raised concern about its broad application as a biopsychologic probe(20). Moreover, the magnitude of RSA is influenced by both respiratory rate and tidal volume (i.e. RSA increases as respiratory rate slows and tidal volume increases). Thus the magnitude of the RSA may be a measure of cardiac vagal activity only when respiratory activity remains stable, which is rarely the case in a clinical setting with infants-particularly during exposure to noxious stimuli.
To account for the empirical limitations of spectral analysis of HR variability, the mathematical technique, TFA, was used to derive specific measures of parasympathetic and sympathetic modulation of HR from the relationship between short-term changes in HR (RSA) and respiratory activity(21,22,18). Use of transfer function analysis of HRV and respiratory activity has demonstrated a maturational increase in sympathetic modulation of HR during quiet sleep in healthy preterm infants(23) and the autonomic effects of both spinal anesthesia in former premature infants during minor surgery(21) and halothane anesthesia in healthy full-term infants during the first year of life(22).
The aim of this study was to examine specific autonomic control mechanisms that underline cardiac response to an acute noxious event. We hypothesized that in response to a noxious event, sympathetic HR modulation would increase and parasympathetic would decrease and that this response would change between 4 and 8 months of age, reflecting a maturation of the autonomic system. To study the changes in cardiac autonomic modulation that occur with acute noxious stimulus and with age during infancy, the relationship between respiratory activity and short-term variations of HR (i.e. RSA) was quantified in a longitudinal study of term infants who underwent a finger lance blood collection at 4 months and again at 8 months of age.
METHODS
Subjects. With approval from the UBC Ethics, BC Children's Hospital, and BC Women's Hospital Scientific Review Committees, 24 healthy term infants were recruited at birth and were studied at 4 months and again at 8 months as part of a larger study of iron metabolism and infant development in healthy term infants and former low birth weight infants. None of these term infants was taking medication, had neurologic, cardiac, or respiratory problems; or had a history of prolonged exposure to pain.
Procedure and physiologic signal acquisition. Each infant was used as his or her own control. The study was conducted in a specially designed room in the Biobehavioral Research Unit in the Centre for Community Child Health Research. The infant was seated on his/her mother's lap, and recording began when the infant was in an awake, alert, and noncrying state(24). Three standard surface chest electrodes were used to produce continuous ECG, and a two-belt microprocessor-controlled inductance plethysmograph system was used to produce a respiratory signal (Respiratrace-Plus, NonInvasive Monitoring Systems, Miami, FL). Both signals were digitally sampled to disk at 360 Hz using a personal computer-based data acquisition system(25). Calibration of the respiratory signal was performed using a previously described algorithm(26), assuming an infant tidal volume of 7 mL/kg. Respiratory volumes were normalized to a standard body surface area of 1.73 m2 to enable comparisons between previous adult and infant respiratory data. One lead of surface ECG and respiratory activity was recorded continuously during baseline, lance, and recovery periods. The lance procedure was done by one of three technicians and was done with one lance of the second finger. The mean handling times did not vary significantly between ages (138 s at 4 months and 132 s at 8 months).
Data analysis. R waves were detected from the sampled ECG and used to form a smoothed instantaneous 4-Hz HR time series(27). The inductance RP was digitally low pass-filtered and decimated to 4 Hz. Segments of HR and respiratory activity (2.2 min each) were selected from 1) the resting baseline period within 5 min of the lance, 2) a lance period starting within 20 s after the finger prick blood collection, and 3) a recovery period within 7 min after the lance. The epoch selection criteria were based on(28) quantitative signal stationarity, the presence of a stable behavioral state, and the absence of gross movement artifact.
Power spectral estimates of HR were quantified using the area (power) of the spectrum in a low-frequency region (LF: 0.04-0.15 Hz) and a high-frequency region (HF: 0.15-0.80 Hz), as well as by the ratio of LF and HF power (LF/HF), as previously described(18). Similar measures of respiratory activity were tabulated from the RP spectrum to yield total RP (LF-RP + HF-RP).
To determine the contribution of both sympathetic and parasympathetic components to HR modulation, the effect of respiratory activity on HR was assessed using transfer function analysis, as previously reported in infants and adults(22,18). Autospectra of the HR and respiratory signals and the cross-spectrum between them were estimated for each 128-s (512 point) segment as previously described(29). The complex transfer function, or frequency response, between respiratory activity and HR was quantified using the cross-spectral method to yield magnitude (gain) and phase components. A squared coherence spectrum was also computed to define the degree of the linear relation between respiratory activity and HR. The coherence varied between 0 and 1.
The average coherence weighted transfer gains of the 2.2-min segments of data were used to assess changes in cardiac parasympathetic and sympathetic control. Using this technique, previous work during pharmacologic treatment of adults with either atropine while upright, or propranolol while supine, demonstrated transfer gain and phase plots characteristic of pure parasympathetic and pure sympathetic modulation of HR(18), respectively. A pure sympathetic HR response (during standing with atropine) was characterized by a reduced gain at frequencies >0.01 Hz and a phase delay. In contrast, under pure vagal conditions (supine plus propranolol), the HR responses were characterized by higher gain at all frequencies and no phase delay.
Statistical analysis. The mean and SD of the HR, respiratory activity, and power spectra for each data segment were calculated. A repeated-measure analysis of variance was used to compare outcome measures across study periods. Post hoc comparisons were done where appropriate using Tukey tests. A difference was considered statistically significant for p values less than 0.05. For the purposes of display, group average transfer function estimates of both gain and phase from each experimental epoch were computed as previously described(18).
RESULTS
A group of 24 healthy infants were studied at 4 months (mean age 4.1 ± 0.2), 20 of whom returned at 8 months (mean age 8.4 ± 0.3). Four infants did not return at 8 months for parental reasons. All infants underwent the finger lance blood collection without complications. Mean gestational age at birth was 39.9 ± 0.9 wk, Apgars 7.5 and 9.0 at 1 and 5 min, respectively. Mean birth weight was 3641 ± 416 g.
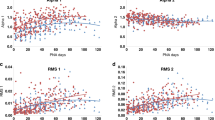
Mean HR. Mean HR increased significantly from baseline to lance period and declined again in recovery periods at both ages (Fig. 1) [F = 83.6, df(2, 46), p < 0.01; and F = 36.1, df(2, 38), p < 0.01, respectively]. However, the change in mean HR from baseline to lance was significantly different at 4 and 8 months (50 vs. 31%, respectively) [df(19, 1), t = 3.872, p = 0.001].
Power spectral estimates. At 4 months, after the lance, bot LF and HF power decreased significantly [F = 3.6, df(2, 46), p = 0.035; and F = 6.9, df(2, 46), p = 0.002, respectively] from baseline and increased again in the recovery period (Table 1). The ratio LF/HF also decreased. Although the differences were not significant across all periods [F = 0.5, df(2, 46), p = 0.61], the changes reflected a greater decrease in LF than in HF power (Fig. 2). Finally, total RP increased from baseline with the lance and decreased in the recovery period [F = 9.71, df(2, 46), p < 0.01] (Fig. 3).
In contrast, at 8 months, LF increased from baseline and remained elevated in the recovery epoch. The changes in HF were more similar to 4 months, a decrease after the lance and an increase again in the recovery epoch. However, differences across the three epochs were not significant [F = 2.6, df(2, 38), p = 0.09; and F = 1.1, df(2, 38), p = 0.35, respectively] (Table 1). Again, in contrast to 4 months the LF/HF ratio increased significantly from the baseline with the lance and remained elevated in the recovery epoch [F = 3.68, df(2, 38), p = 0.04], reflecting a relatively greater increase in LF than in HF. As at 4 months, total RP increased with lance and declined in the recovery period [F = 5.6, df(2, 38), p < 0.01] (Fig. 3).
Transfer function estimates of respiratory sinus arrhythmia: 4 months. During the baseline period, the transfer gain was high at all frequencies. The phase began at 0° at 0 Hz and decreased slightly with increasing frequencies, showing a predominance of vagal cardiac modulation in the baseline state.
In response to the lance, transfer gain decreased significantly across all frequencies but became very low in the high frequency range. The phase also fell more consistently with increasing frequency, suggesting a marked withdrawal of parasympathetic and a slight increase of sympathetic cardiac modulation. The changes in LF and HF transfer gain in Fig. 4 are consistant with a significant shift away from parasympathetic and toward sympathetic HR control in both groups. In the recovery epoch, gain increased again across all frequencies whereas phase remained and actually became close to 0° at frequencies up to 0.5 Hz, consistent with a return of sympathetic and parasympathetic modulation to near baseline levels.
Transfer function estimates of respiratory sinus arrhythmia: 8 months. In contrast to the 4 months transfer gain, the 8 months low and high frequency transfer gain response was significantly less at all frequencies (F = 4.8, df(2, 38), p = 0.014; F = 4.8, df(2, 38), p = 0.014) (Fig. 4). During the baseline condition, transfer gain began high at all frequencies (Fig. 4), whereas phase began at 0° at 0 Hz and stayed near or above 0° with increasing frequency, again suggesting a vagal predominance.
After the lance, transfer gain decreased in the HF region but stopped at levels higher than those at 4 months and remained stable in the LF region. Phase, however, had a marked decrease with increasing frequency. These findings suggest an increase of cardiac sympathetic modulation but a smaller concomitant withdrawal of vagal modulation than at 4 months.
In the recovery period, transfer gain increased again across all frequencies (although somewhat less in the HF region) and the negative phase slope remained, indicating a return of parasympathetic modulation and a persistence of the sympathetic response.
In summary, all the transfer function and power spectral results indicated a greater reduction of cardiac vagal modulation with the painful stimulus in the 4-month group, and a larger sustained increase of cardiac sympathetic modulation in the 8-month group.
DISCUSSION
There are two primary findings from this study. First, a response to an acute noxious stimulus appeared to produce an increase in respiratory-related sympathetic HR control and a significant decrease in respiratory-related parasympathetic control at both 4 and 8 months. Second, there was a developmentally-based shift in the responses, such that the increase of sympathetic modulation is larger at 4 months and more sustained at 8 months of age (LF/HF and in Figs. 2 and 4), and the parasympathetic withdrawal is smaller (reduced increased in HR, HF, in Figs. 1,2 and 4) at 8 months of age. These findings further suggested that the larger increase of mean HR in the 4-month-old infants was caused by a larger reduction of vagal activity.
Using HR spectral data alone, the reduction in LF, HF, and their ratio at 4 months might have been interpreted as a decrease in both sympathetic and parasympathetic HR modulation, a conclusion that is possibly inconsistent with either the changes in mean HR or the expectation of sympathetic activation with the noxious stimulus. Furthermore, these results would have contrasted with the 8-month findings where LF increased and HF remained unchanged, suggesting that sympathetic modulation increased whereas parasympathetic modulation remained stable after the lance. Thus, inclusion of the influence of respiration and the transfer functions to compute transfer gain and phase provided results that are more consistent with both the expected physiology and the nonspectral (i.e. mean HR) data than LF, HF, and their ratio.
Similarly, in the recovery period the HR spectral power data alone might have been misleading. At 4 months, LF power and LF/HF ratio increased, presumably reflecting a return of sympathetic cardiac modulation, whereas HF made a nonsignificant increase from the prick period, also reflecting a return of vagal modulation. Using these HR spectral data alone, such reductions might be interpreted as an increase of parasympathetic and sympathetic HR modulation. However, we also found a significant increase in respiratory signal power. Because LF and HF fluctuations are strongly influenced by both the frequency and amplitude of respiratory activity(30,31), such changes in respiratory activity must be accounted for when interpreting spectral results. When the transfer function between respiration and HR was used to account for the effects of changes in respiratory activity, sympathetic HR control decreased during recovery, whereas parasympathetic control increased significantly both at 4 months and again at 8 months.
These findings have a number of implications. First, with age, there was an increased capacity to modulate a response to a painful event. Second, as noted above, with use of spectral data alone, the reductions in LF and HF at 4 months would have been inconsistent with either changes in mean HR or the clinical expectation that sympathetic arousal follows a noxious event. In contrast, inclusion of respiratory activity to compute transfer gain yielded results more consistent with an expected sympathetic arousal. These conclusions demonstrate the importance of accounting for the influence of respiratory activity and distinguishing between components of HR variability that are respiratory-dependent and those that are respiratory-independent (LF and HF).
In summary, the primary finding of this study is that acute noxious stimuli in healthy infants appears to produce a marked reduction in respiratory-related parasympathetic HR control and a marked increase in respiratory-related sympathetic HR control. Furthermore, with increasing age during infancy, the sympathetic and parasympathetic changes appear to be less intense but more sustained.
Autonomic development and HR control. It is difficult to compare our data with previous studies of the developmental influences on HR control in infancy or cardiac responses to noxious stimuli. Overall, mean HR decreases with age during infancy(13). This decline may be caused by maturation of parasympathetic cardiac modulation; however, nonautonomic influences such as physiologic anemia, metabolic rate, and body weight may also contribute to an increased HR early in life and gradually decrease with time. Furthermore, the changing pattern of HR variability during the first 6 months of life may not reflect a linear pattern of development(35,36). During all sleep-awake states, the extent of RSA appears to increase from 1 to 6 months, after an initial decrease during the first month of life. Low frequency HR variably follows a similar pattern; however, it appears to be more pronounced in quiet sleep(36), suggesting a maturation of both sympathetic and parasympathetic cardiac modulation. How these changes reflect changing responses to noxious stimuli or reflect maturation of the infant's autonomic system is unclear. It has been observed that the sympathetic nervous system matures earlier in fetal life than does the parasympathetic system, coupled with increasing parasympathetic contribution to HR control after birth in both animals(32) and human infants(15,16,33) Similarly, using HR spectral data, sympathetic and parasympathetic cardiac modulation appears to increase with age in preterm(23) and term infants(34–36). It remains unclear how such autonomic maturation influences the cardiac response to noxious stimuli. Recent work has demonstrated that preterm and term neonates appear to be less able to generate responses to noxious stimuli compared with older infants(37). Across all gestational ages, mean HR clearly increases in response to painful procedures(1,8,11), but there appears to be a developmental shift in mean HR response with time(12,17,23). How this shift reflects change in the autonomic nervous system has yet to be determined. Conceivably, a change of autonomic cardiac control that occurs with development may reflect changes in both the absolute levels and the balance between components of the system, as well as the capacity of the autonomic nervous system to modulate responses to noxious stimuli in increasingly more varied and complex ways. Finally, it also remains to be determined how autonomic development observed in this study relates to the substantial social/behavioral maturation that also occurs during the same period of infancy.
Limitations. A number of study limitations should be mentioned. Use of transfer function analysis is dependent on the amplitude of the RSA being a function of changes in tidal volume and respiratory rate. Although this link has been established in adults(31,38), the effect is not linear and has yet to be confirmed in infants. In this study, even with all infants in a similar behavioral state at baseline, the underlying autonomic arousal may have not have been similar levels among all infants before the lance. Therefore, the changes in autonomic arousal may have varied as a function of the starting level of arousal as much as a function of the noxious stimulus. A larger sample size in future studies will help in investigating this latter issue. Similarly, even by seeking to study infants in similar behavioral states, the adverse influence of body movement and cry artifact can only be reduced but not eliminated. We attempted to reduce the confounding effect of crying by selecting postlance periods that were close but not directly associated with the lance itself (i.e. 20 s after the lance). Finally, although we studied cardiac autonomic changes that reflect responses to noxious stimuli, our outcomes should not be considered a direct measure of infant pain per se. Further work is needed to determine the cardiac autonomic responses to specific noxious and nonnoxious stimuli.
In conclusion, healthy term infants experiencing an acute noxious event responded with an increase in sympathetic cardiac modulation and a withdrawal in parasympathetic modulation. There also appeared to be a developmentally based shift with less pronounced but more sustained sympathetic and parasympathetic responses in 8-month-old infants compared with 4-month-old infants, reflecting a maturation of autonomic cardiac response to a noxious stimulus. This study provides a methodology for studying cardiac autonomic responses to a noxious event in infants and its developmental course.
Abbreviations
- HRV:
-
heart rate variability
- RSA:
-
respiratory sinus arrhythmia
- LF:
-
low frequency
- HF:
-
high frequency
- RP:
-
respiratory power
- As:
-
sympathetic gain factor
- Ap:
-
parasympathetic gain factor
References
Porter FL, Porges SW, Marshall RE 1988 Newborn pain cries and vagal tone: parallel changes in response to circumcision. Child Dev 59: 495–505.
Anand KJS, Aynsley-Green A 1988 Measuring the severity of surgical stress in newborn infants. J Pediatr Surg 23: 297–305.
Anand KJS 1990 Neonatal stress responses to anesthesia and surgery. Clin Perinatol 17: 207–214.
Randich A, Maixner W 1984 Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 8: 343–367.
Owens ME, Todt EN 1984 Pain in infancy: neonatal reaction to heel lance. Pain 20: 77–86.
Beaudoin CA, James M, McAllister M 1991 The physiological response of premature infants to heelstick blood sampling. J Pain Sympt Manag 6: 193
Grunau RE, Oberlander T, Hoslti L, Whitfield M 1998 Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 76: 277–286.
McIntosh N, Van Veen L, Brameyer H 1993 The pain of heel prick and its measurement in preterm infants. Pain 52: 71–74.
Johnston CC, Stevens B, Craig KD, Grunau RVE 1993 Developmental changes in pain expression in premature, full-term, two- and four-month-old infants. Pain 52: 201–208.
Lewis M, Thomas D 1990 Cortisol release in infants responding to inoculation. Child Dev 61: 50–59.
Johnston CC, Stevens BJ, Yang F, Horton L 1995 Differential responses to pain by very premature neonates. Pain 61: 471–479.
Craig KD, Whitfield MF, Grunau RV, Linton J, Hadjistavropoulos HD 1993 Pain in the preterm neonate: behavioural and physiological indices. Pain 52: 287–299.
Woodrow-Benson D 1995 The normal electrocardiogram. In: Emmanouilides GC, Allen HD, Riemenschneider TA, Guttgesell HP (eds) Heart Disease in Infants Children and Adolescents. Williams and Wilkins, Baltimore, 160
Lagercrantz H, Edwards D, Henderson-Smart D, Hertzberg T, Jeffery H 1990 Autonomic reflexes in preterm infants. Acta Paediatr Scand 79: 721–728.
Finley JP, Hamilton R, MacKenzie MG 1984 Heart rate response to tilting in newborns in quiet and active sleep. Biol Neonate 45: 1–10.
Dohi S, Naito H, Takahashi T 1979 Age-related changes in blood pressure and duration of motor block in spinal anesthesia. Anesthesiology 50: 319–23.
Johnston CC, Stevens BJ, Yang F, Horton L 1996 Developmental changes in response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol 38: 438
Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ 1991 Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol 261:H1231–H1245.
Fox NA, Porges SW 1985 The relation between neonatal heart period patterns and developmental outcome. Child Dev 59: 28–37.
Litvack DA, Oberlander TF, Carney LH, Saul JP 1995 Time and frequency domain methods for heart rate variability analysis: a methodological comparison. Psychophysiology 32: 492–504.
Oberlander TF, Berde CB, Lam KH, Rappaport LA, Saul JP 1995 Infants tolerate spinal anesthesia with minimal overall autonomic changes: transfer function analysis of respiratory sinus arrhythmia in former premature infants undergoing hernia repair. Anesth Analg 80: 20–27.
Oberlander TF, Berde CB, Lam KH, Saul JP 1996 Halothane causes withdrawal of cardiac sympathetic modulation in infants: transfer function analysis of respiratory sinus arrhythmia. Pediatr Res 40: 710–717.
Hanna BD, Saul JP, Cohen RJ, Stark AR 1990 Transfer function analysis of respiratory sinus arrhythmia: developmental changes in sleeping premature infants. Circulation 82( suppl III): 334.
Prechtl HF 1974 The behavioral states of the newborn infant (a review). Brain Res 76: 185–212.
Boston Medical Technologies 1996 HR View Software, Boston MA.
Brouillette RT, Morrow AS, Weese-Mayer DE, Hunt CE 1987 Comparison of respiratory inductive plethysmography and thoracic impedance for apnea monitoring. J Pediatr 111: 377–383.
Berger R, An efficient algorithm for spectral analysis of heart rate variability. IEEE Transactions on Biomedical Engineering 33: 900–904.
Johnston CC, Stevens BJ, Yang F, Horton L 1996 Developmental changes in response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol 38: 438–445.
Berger RD, Saul JP, Cohen RJ 1989 Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am J Physiol 256:H142–H152.
Angelone A, Coulter NA 1964 Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol 19: 479–482.
Eckberg DL 1983 Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol 54: 961–966.
Nuwayhid B, Brinkman CR, Su C, Bevan JA, Assali NS 1975 Development of autonomic control of fetal circulation. Am J Physiol 228: 337–344.
Chatow U, Davidson S, Reichman BL, Akselrod S 1995 Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res 37: 294–302.
Snidman N, Kagan J, Riordan L, Shannon DC 1995 Cardiac function and behavioral reactivity during infancy. Psychophysiology 32: 199–207.
Harper RM, Walter DO, Leake B, Hoffman HJ, Sieck GC, Sterman MB, Hoppenbrouwers T, Hodgman J 1978 Development of sinus arrhythmia during sleeping and waking states in normal infants. Sleep 1: 33–48.
Schechtman VL, Harper RM, Kluge KA 1989 Development of heart rate variation over the first 6 months of life in normal infants. Pediatr Res 26: 343–346.
Anand KS, Grunau RE, Oberlander TF 1997 Developmental character and long-term consequences of pain in infants and children. Child Adolesc Psychiatr Clin North Am 6: 703–724.
Hirsch JA, Bishop B 1981 Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol 241:H620–H629.
Acknowledgements
The authors thank Colleen Fitzgerald, Ann-Louise Ellwood, Jacqueline Smit Alex, Herb Chan, and the staff of the Newborn Nursery at BC Women's Hospital for their invaluable contributions.
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from the British Columbia Health Research Foundation.
Rights and permissions
About this article
Cite this article
Oberlander, T., Grunau, R., Pitfield, S. et al. The Developmental Character of Cardiac Autonomic Responses to an Acute Noxious Event in 4- and 8-Month-Old Healthy Infants. Pediatr Res 45, 519–525 (1999). https://doi.org/10.1203/00006450-199904010-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00011
This article is cited by
-
The newborn infant parasympathetic evaluation index for acute procedural pain assessment in preterm infants
Pediatric Research (2021)
-
Newborn infant parasympathetic evaluation (NIPE) as a predictor of hemodynamic response in children younger than 2 years under general anesthesia: an observational pilot study
BMC Anesthesiology (2019)
-
NIPE is related to parasympathetic activity. Is it also related to comfort?
Journal of Clinical Monitoring and Computing (2019)