Abstract
To date, quantitative studies of the inherent characteristics of the developing airway wall have required excision of an airway segment or surgical creation of an isolated segment. We hypothesized that airway wall characteristics, at various collapsing pressures, and attendant changes in stiffness after smooth muscle stimulation could be quantitated bronchoscopically from airway pressure-area relationships. Neonatal lamb tracheal segments (n = 12) were suspended over hollow mounts, in a buffer-filled chamber, and subjected to a range (0 to -4.0 kPa) of pressures to determine wall stiffness under collapsing forces before and after stimulation of the trachealis with methacholine. Luminal images were recorded through a 3.6-mm flexible bronchoscope under the same conditions, subsequently corrected for distortion, and a cross-sectional area was quantitated. Both pressure-volume and pressure-area relationships detected significant changes in airway wall stiffness after methacholine administration (p < 0.002), and the magnitude of change was similar between methods. These data suggest that quantitative flexible bronchoscopy can be used clinically in the intact airway to assess wall stiffness.
Similar content being viewed by others
Main
Abnormal central airway collapsibility is an important cause of obstruction in infants and young children(1–3). Congenital tracheomalacia and bronchomalacia presumably result from a weakness of the structural elements of the airway wall(4–6). They are often associated with other anomalies such as tracheoesophageal malformations(7), but may exist as isolated entities(6, 8). Acquired central airway collapse arises after trauma and deformation associated with positive pressure ventilation or prolonged compression(9–12), especially as a complication of bronchopulmonary dysplasia or congenital heart disease(3, 13–15).
Immature airways are inherently compliant structures, but they become less deformable with maturation(16–18). In infants and young children, elevation of pleural pressure accompanying increased expiratory effort can cause central airway collapse and be mistaken for true tracheomalacia or bronchomalacia. In contrast, neural or pharmacologic stimulation of airway smooth muscle decreases airway compliance and collapsibility throughout the developmental spectrum(19–21).
A variety of imaging techniques has been used to diagnose central airway collapse in infants, including airway fluoroscopy(2), rapid cine-computerized tomography(3), and airway endoscopy(6, 8, 14, 22). None of these studies, however, has correlated the degree of collapse with the amount of pressure applied across the airway wall.
Estimates of tracheal wall compliance have been made successfully in adults using an acoustic area reflection technique(23). The technique requires the subject's cooperation, and so is not amenable for use in infants. Thus, previous measurements of maturational or interventional changes in wall stiffness required the formation of isolated segments from excised airways, or the surgical creation of bypassed isolated segments in animal preparations to detect such changes(16, 18, 20, 21, 24, 25). To date, wall stiffness has not been measured in the intact immature airway. We were interested, therefore, in developing a method that would lend itself to such measurements, so that changes in central airway mechanics could be interpreted within the framework of maturational or disease processes.
The most accurate method of identifying central airway collapse is by direct visualization of the airways. Currently, bronchoscopy gives only a qualitative or semi-quantitative(8, 14, 22, 26) impression of airway collapsibility. Recent studies have demonstrated that the visual “barrel”-type distortion present in fiberoptic endoscope images can be mathematically corrected so that sizing of objects or airways can be accomplished accurately(27, 28). We hypothesized that the inherent mechanical characteristics of the airway wall could be accurately quantitated using a flexible bronchoscope to measure airway cross-sectional area under conditions of varying PTM values and smooth muscle tone. To test this hypothesis, we performed pressure-volume determinations over a range of collapsing pressures in neonatal isolated tracheal segments, and compared them with pressure-area measurements. Furthermore, to assess the accuracy with which pressure-area measurements reflect a change in airway stiffness, we measured changes in pressure-volume and pressure-area relationships resulting from the administration of MCh, an airway smooth muscle agonist. This study describes a technique that ultimately can be adapted to study structural characteristics of the intact airway.
METHODS
Tracheae from 12 neonatal lambs (3.28 ± 0.15 (SE) kg) were used for this study. Animals were sedated with pentobarbital (20 mg/kg i.p.) and killed by lethal injection of potassium chloride. Segments from either the lower extrathoracic or the proximal intrathoracic portion of the trachea, at least 3 cm in length, were carefully dissected from surrounding connective tissues and removed immediately after death of the lamb. A small ring of tissue was cut from the end of the tracheal segment, and the internal diameter of the tracheal ring was measured with calipers.
The tracheal segment was placed in a 200-mL Lucite chamber filled with oxygenated KBS warmed to 37 °C and maintained at a pH of 7.4 ± 0.05. The apparatus used was adapted from that developed by Penn et al.(21) (Fig. 1). The apparatus was designed to be used as either a volume or pressure plethysmograph. The setup allowed for variation of pressure around the airway while the lumen of the trachea was available for visualization, as well as for alteration of Plum values. The chamber had both a fixed and a sliding hollow mount, a port for attachment of a micropipette, and an inflow/outflow port connected to a pressure transducer (Gould P23 dB) to measure Pc. Both the micropipette and pressure transducer were placed at the same height as the hollow mounts. A port was placed on the fixed mount for injection or withdrawal of air, and another pressure transducer was attached to a port on the sliding mount to measure Plum. In this way, PTM (Plum- Pc) could be measured. All ports were outfitted with stopcocks for injection/withdrawal of fluid or air. For pressure-area studies, the chamber was used as a pressure plethysmograph, where PTM was varied by adjusting Pc while maintaining the airway lumen at atmospheric pressure. In contrast, for pressure-volume determinations, the chamber was used as a volume plethysmograph, where PTM was altered by adjusting Plum while keeping Pc open to the atmosphere.
Schematic diagram shows top view of apparatus used for pressure-volume and pressure-area studies. (A) Lucite chamber;(B) fixed hollow mount; (C) sliding hollow mount;(D) pressure transducer measuring Pc; (E) pressure transducer measuring Plum; (F) port for injection or withdrawal of air; (G) outflow port; (H) inflow port;(I) reservoir containing warmed, oxygenated KBS; (J) peristaltic pump; and (K) port for horizontally placed micropipette.
The trachea was tied around the barrels of the fixed and sliding mounts with -inch umbilical tape, excluding the minimum amount of airway necessary to prevent the trachea from slipping off the mount. The mounted airway was stretched minimally to take up any slack, and the sliding mount was held fixed for the duration of the experiment. Warmed KBS, through which 95% O2/5% CO2 was continuously bubbled, was circulated through the chamber for 30 min to allow the viscoelastic forces within the tissues to return to a resting state after the airway had been manipulated, and to allow for equilibration with any pressure forces within the circuit. The trachea was mounted within 1.2 cm of the chamber top and the Plum was zeroed to atmosphere after the chamber was fluid filled, to avoid the effects of hydrostatic pressure. Absence of a persistent hydrostatic effect was confirmed by a lack of any volume change when the chamber was opened to the horizontally mounted micropipette.
All airways were studied using the following protocol: 1) baseline pressure-area determinations, 2) 5-10-min recovery period,3) baseline pressure-volume measurements, 4) 5-10-min recovery period, 5) MCh administration with 10-min equilibration,6) MCh pressure-volume determinations, and 7) MCh pressure-area measurements. During recovery periods, KBS was circulated through the apparatus. This sequence allowed for comparison of pressure-volume and pressure-area determinations after the administration of the same aliquot of MCh, and also allowed us to measure changes in resting tracheal volume after MCh administration.
Pressure-area studies. The Lucite chamber was zeroed to atmospheric pressure and sealed. A three-way Y-adapter with a slit diaphragm(Bodai Suction-Safe TM, Swivel Y, Sontek Medical, Hingham, MA) was placed on the fixed mount to support the bronchoscope. A standard 3.6-mm (outside diameter) pediatric flexible fiberoptic bronchoscope (Olympus model BF-3C30, Olympus America Inc., Columbia, MD) with a 1.2-mm suction channel was inserted through the slit diaphragm and advanced to the end of the hollow mount. The tip of the bronchoscope was placed so that the tracheal image was centered within the field of view, to minimize the possibility that the bronchoscope would contact the wall and decrease area changes during tracheal collapse. All pressure transducers were connected to a Grass model 7 multichannel recorder(Grass Instrument Co., Quincy, MA), and Pc and Plum were monitored throughout the entire experiment. A video camera was attached to the head of the bronchoscope and continuous real-time images of the airway lumen were viewed on a monitor and recorded on a VHS video recorder. A bronchoscopy brush, calibrated in 2.5-mm increments, was passed through the suction channel of the scope and into the field of view to determine image distance from the tip of the bronchoscope.
After measurements were made at atmospheric pressure, the brush was withdrawn into the suction channel and KBS was injected into the chamber to create a collapsing pressure of 0.5 kPa (-5 cm H2O) (PTM -0.5 kPa). Images were recorded and the brush was again advanced into the field of view to confirm image distance. Whenever possible, the bronchoscope was flexed or extended so that the brush contacted the airway mucosa in two places. The scope was not rotated so that inadvertent advancement and change in image perspective could be minimized. If the brush contacted the mucosa in only one spot, the corresponding cartilage ring was used to establish image distance. Pc was increased stepwise to achieve PTM values of -1.0, -2.0,-3.0, and -4.0 kPa (-10 to -40 cm H2O), and imaging sequences were repeated at each pressure. KBS was then withdrawn from the chamber in increments to achieve the same Pc values until atmospheric pressure was reached, while images were recorded at each pressure level.
Pressure-volume studies. The bronchoscope was removed and both ends of the tracheal mount were plugged, effectively creating a closed tracheal segment. A 1.0-mL micropipette calibrated in 0.01-mL divisions was mounted to the chamber horizontally at tracheal level and filled with KBS. A stopcock was opened to allow communication between the micropipette and the chamber. Air was withdrawn from the tracheal lumen in increments to generate PTM values of -0.5, -1.0, -2.0, -3.0, and -4.0 kPa (-5 to -40 cm H2O). The volume change noted in the micropipette reflected the volume change of the trachea. Air was then injected in the same pressure increments until atmospheric pressure was reached, and volume changes in the micropipette were recorded at each pressure.
After a recovery period, the chamber was again sealed. The micropipette port was opened, and MCh (final concentration 10-3 M) was injected into the chamber and allowed to equilibrate for 10 min. Any change in resting tracheal volume resulting from smooth muscle contraction was reflected in a change in volume in the micropipette. All pressure-volume and pressure-area measurements were then repeated.
Image analysis. The recorded video images were replayed and captured by a computerized video frame grabber and image analysis system(Global Lab Image 3.1, Data Translation, Inc., Marlboro, MA). The system was calibrated using 10- or 14- points/cm grids to eliminate radial or“barrel”-type distortion. Calibration files were created by fixing the bronchoscope perpendicular to the grid at 0.5, 0.75, or 1.0 cm from the surface. Points on the video image were assigned real-world values using x-y coordinates, and ranged from the center to the periphery of the bronchoscope field. The calibration file matching the measured distance of the airway image from the tip of the bronchoscope was then used to correct for radial distortion and perspective. To confirm the accuracy of this method, the horizontal diameter of each airway was calcualted using the image analysis system, and compared with the actual internal diameter. Tracheal cross-sectional area was then calculated by the image analysis system based on the perimeter outlined by the operator using a computer mouse. At each pressure, five area determinations were made, and the mean value was used for analysis.
Data analysis. The length of the tracheal segment was determined by measuring the distance between the two ties. Resting airway volume was calculated from length and diameter measurements, assuming the nonstressed trachea to be a cylinder(16, 18, 21). Subsequent PTM-related changes in tracheal volume were reflected in a volume change within the micropipette, and were normalized to the resting tracheal volume(16). The resulting specific volume was plotted against collapsing pressure to produce an Caw curve. The change in tracheal volume resulting from MCh stimulation was calculated by subtracting the displaced volume in the micropipette from the resting volume. Specific Caw after MCh was calculated using this new volume.
In a similar fashion, pressure-area calculations were made before and after tracheal stimulation with MCh, using PTM determinations and the computer-assisted calculations of lumen area. Pressure-related changes in cross-sectional area were normalized to baseline area measurements. Pressure-area curves were then plotted for each segment under both conditions. An ACI was calculated by measuring the change in area/resting area for a given change in PTM. For both Caw and ACI determinations, points from-1.0 to 0 kPa (-10 cm H2O to 0 cm H2O), which fell within the linear portion of the ascending compression curve, were used for calculations.
For each airway, the mean of five diameter measurements taken at the same location within the airway segment and computed by the image analysis system was compared with the internal tracheal diameter as determined directly by the caliper measurement made on the unstressed tracheal ring at the beginning of the experiment. To relate the two methods of assessing wall stiffness, we compared the percent change in area or volume under baseline conditions as a function of PTM and of measurement type by two-way repeated measures ANOVA and Newman-Keuls post hoc analysis. To determine how well either method was able to detect changes in wall stiffness, airway compliance and ACI before and after MCh administration each were compared by paired a t test, as was the magnitude of change in Caw or ACI after MCh administration. The magnitude of change in Caw and ACI were also compared with each other by a paired t test. Additionally, because significant central airway collapse is often determined bronchoscopically by a percentage of luminal narrowing from baseline(2, 13, 14), the PTM required to decrease airway cross-sectional area by 50% was calculated by linear interpolation both before and after MCh administration. In three airways, the decrease in cross-sectional area at -4.0 kPa PTM did not exceed 50% of resting area after exposure to MCh, so they were eliminated from this analysis. Significance was determined at a p < 0.05 level.
RESULTS
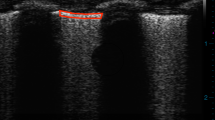
The tracheal segments had a radius of 0.33 ± 0.01 (SE) cm, a length of 2.70 ± 0.19 (SE) cm, and a resting volume of 0.96 ± 0.11 (SE) mL. Application of collapsing pressure to the relaxed tracheal segment typically caused invagination of the pars membranacea at lower collapsing pressures, whereas at higher collapsing pressures the lumen assumed an asymmetric comma or curl formation (Fig. 2). After exposure to MCh, the posterior membrane appeared more prominent but shorter in its transverse dimension under resting conditions. Additionally, when the tracheae were exposed to collapsing pressures, the lumen remained round, and the curl formation was less prominent (Fig. 2). Typical pressure-volume and pressure-area curves are shown in Figures 3 and 4. For both types of measurement, hysteresis was present between descending and ascending limbs of the curves (Fig. 3). Furthermore, the mean values of maximum collapsibility of the unstimulated airway, defined as the percent decrease from baseline in either volume or area at -4.0 kPa PTM, were similar for both methods (-70 ± 4% SE Δvolume; -72 ± 3% SEΔarea) (Figs. 3 and 5).
Bronchoscopic views of a single tracheal lumen at varying PTM before (a-d) and after (e-h) MCh administration. Numbers refer to the PTM (kPa) at which the image was taken. In the unstimulated trachea, as PTM became negative, the posterior membrane invaginated into the lumen (b, -2.0 kPa PTM). As PTM became more negative, the posterior membrane deformed asymmetrically creating a “curl” or comma formation(c, -3.0 kPa PTM and d, -4.0 kPa PTM). After MCh administration, invagination and curl formation of the posterior membrane was markedly diminished at every PTM, and the luminal area was better preserved. The decrease in cross-sectional area from baseline at -4.0 kPa PTM for the unstimulated airway was -79%, whereas after MCh administration it was -51%.
Mean ± SEM values of percent change in volume(open circles) or cross-sectional area (closed circles) normalized to resting values as a function of PTM for 12 tracheae under baseline conditions. Tracings depict pressure changes back toward zero. Two-way repeated measures ANOVA demonstrated a greater change in area than in volume as PTM became less negative (p < 0.001), and post hoc analysis demonstrated significant differences at pressures above -2.0 kPa (*p < 0.05 vs corresponding area value).
Differences did exist, however, between the two measurement methods (Fig. 5). As PTM returned to 0, greater changes were seen in area than in volume (p < 0.001), with measurements at -2.0, -1.0, -0.5, and 0 kPa being significantly different from each other(p < 0.05). At the most negative PTM values, however, both methods detected similar magnitudes of change from the resting condition. Furthermore, as PTM became less negative, the volume was typically lower than resting volume, whereas area measurements were more variable (Figs. 3 and 4).
The calibrated image analysis system was able to correct for image distortion and allowed for accurate sizing of the airway. There was no difference between airway diameters calculated bronchoscopically [0.70± 0.02 (SE) cm] or measured directly with calipers [0.66 ± 0.02(SE) cm]. The coefficient of variation for all area measurements was 3.56± 0.10 (SE)%.
Changes in airway wall stiffness resulting from smooth muscle stimulation were detected by both methods (Fig. 4 and Table 1). Both Caw and the ACI decreased significantly after MCh stimulation. The magnitude of change as measured by both methods was similar. The PTM required to decrease cross-sectional area by 50% was -1.9 ± 0.3 (SE) kPa before and -2.7 ± 0.2 (SE) kPa after MCh stimulation (p< 0.02).
DISCUSSION
Determinants of airway collapse include the PTM across the airway as well as the inherent elastic characteristics of the wall. We were able to quantitate changes in wall characteristics using a technique that does not require the creation of an isolated airway segment. By measuring PTM at the level of the bronchoscopic image, important observations regarding maturational or interventional effects on central airway function can now be quantitated in the intact airway.
The acoustic area reflection technique has been used in older children and adults to measure airway cross-sectional area as a function of distance from an acoustic impulse in the intact airway(23, 29–31). The technique requires cooperation of the subject, but has been successfully performed in a subject as young as 6 y old(31). The technique has also been coupled with esophageal manometry to calculate approximate values of tracheal compliance in normal adults during a slow vital capacity maneuver(23). Because Plum was not measured directly, the compliance values were based on the assumption that Plum was atmospheric and constant throughout the airways. In contrast, the present technique will allow for local determination of PTM in the intact airway under a variety of circumstances (quiet or forced exhalation) and should be amenable for use in infants, because it does not require cooperation of the subject.
Many investigators have used qualitative means to describe abnormal central airway collapse endoscopically. Presence and severity of tracheomalacia or bronchomalacia have been based on an estimated percent decrease in area of the lumen during exhalation(2, 13, 14). Rozycki et al.(22) assessed the amount of luminal narrowing seen bronchoscopically by calculating cross-sectional area ratios from noncalibrated images of airways at the points of greatest and least narrowing. None of these studies, however, accounted for the infant's degree of expiratory effort and the compressing pressure applied to the airway. In healthy infants with no history of wheeze or airway obstruction, exertional expiratory effort associated with crying or struggling can cause a 50% decrease in the tracheal lumen(32). In our preparation, the amount of pressure required to decrease luminal area by 50% was only 1.9 kPa. Thus, apparently significant central airway collapse seen bronchoscopically may merely be a manifestation of increased compressing pressure.
Both open tube (“rigid”) bronchoscopy and flexible fiberoptic bronchoscopy are available for the study of pediatric airways. Rigid bronchoscopy must be performed while the patient undergoes general anesthesia. Often, the child receives assisted ventilation during the procedure. Consequently, the patient's effort of breathing is decreased and exhalation may be completely passive. Thus, many cases of central airway collapse can be underdiagnosed using this method. In contrast, if flexible bronchoscopy is performed using conscious sedation, then the patient breathes spontaneously and must breathe around the bronchoscope, as well. If sedation is inadequate, or if the size of the bronchoscope is large relative to the airway under examination, the patient may breathe with considerably more effort than usual, causing airway PTM to become negative during exhalation. In this situation, airway collapse, not present under normal circumstances, may be noted. Additionally, if the child has significant small airway disease, central airway collapse may also be misdiagnosed. In this situation, the narrowing may not represent a true weakness of the airway wall, but actually reflect the balance of physiologic forces applied to a compliant structure.
To simplify our study design, we chose to keep the tracheal lumen open to atmosphere when we performed pressure-area determinations. However, we have been able to measure pressures accurately via a vinyl catheter (0.5 mm inside diameter, 0.7 mm outside diameter; Becton Dickinson, Rutherford, NJ) fashioned with a side hole and passed through the suction channel of the bronchoscope. Thus, our preparation does not rely upon creation of a closed segment to measure Plum as have other studies which used a bronchoscope to detect compressive airway closure(26) or changes in luminal diameter after smooth muscle contraction(33) in excised bronchi.
We used an isolated tracheal segment so that pressurevolume relationships could be measured to validate our bronchoscope technique. Our values of tracheal compliance under resting and stimulated conditions are similar to those previously reported(25). Additionally, for both methods, collapsing and reinflation curves displayed characteristic hysteresis loops, as previously described(16–18, 25, 34). The data presented represent measurements taken as the tracheal tissues returned to the 0 PTM point after deformation. Analysis of data from airways as they were deformed away from 0 PTM, however, yielded similar results; only the percent change in Caw after MCh administration (-20.3 ± 6.4%) was different from the values presented in Table 1(p = 0.044). When the magnitude of response to MCh stimulation was compared by both methods in this subsequent analysis, the percent change in ACI was significantly greater than was the percent change in Caw(p = 0.047).
The lack of difference detected in the magnitude of the change in Caw and ACI after MCh stimulation between the two methods of measurement as tissues returned to the 0 PTM point may have resulted from variability in the group response of the tissues. At baseline, variability of Caw was approximately 10%, a value similar to previously reported findings(20, 21, 34). Furthermore, baseline variability of the ACI measurements was also within this range (13%) of reported values. The variability in the degree of airway rigidity after muscular contraction, as expressed by the percent change (19% variability in percent change for Caw measurements and 22% for ACI measurements) is also consistent with other observations(20, 34). Variability in the measurement techniques themselves stems from limitations in resolution of pressure and volume measurements, and resolution of the video image and image distance. Video images were not analyzed in a blinded fashion with respect to the pressures applied, because the dramatic changes in shape of the intraluminal area as collapsing pressures were increased made complete blinding difficult if not impossible. Distance from the bronchoscope tip as determined by the brush was determined either by one person (H.B.P.) or by general agreement of two people (H.B.P. and I.T.). The range over which images were measured was small (between 7.5 and 10 mm), so that differences in perspective were minimized. For this study, one investigator (H.B.P.) performed all area determinations. In subsequent studies, the interobserver variability of cross-sectional area measurements was <7%. We believe that the contribution of these measurement limitations to the variability of our data were small compared with the inherent variability of the tissues studied.
There are important differences between the Caw and ACI measurements we made. Pressure-related volume changes in our isolated tracheal segment represent contributions over the entire segment (i.e. uniform pressure distribution along the airway). The compliance value obtained by this method is an average change in volume and pressure along the length of the preparation. In contrast, area measurements were taken at a focal point along the airway, and reflect local tracheal area changes. Deflection at the mid-point of the preparation is greatest, whereas deformation near the fixation points is lowest. It would not be possible to measure areas accurately distal to the compressed region of the airway, because the physical presence of the bronchoscope would alter distal measurements by contacting the wall. In addition, it cannot be assumed that the airway distal to the narrowing widens symmetrically compared with the airway proximal to the narrowing. Furthermore, the clinical grading of severity of dynamic airway narrowing is based on the change in airway area at the narrowest point as viewed bronchoscopically. For these reasons, we did not measure areas all along the airway segment, but rather chose the middle of the segment where changes would be greatest.
Length of the segment between points of fixation can dramatically affect changes in airway dimensions. We used a paired analysis, however, so that the tracheal length used for volume and area measurements was identical. We also minimized length-related artifacts in our measurements by using a group of airways of similar length. Nevertheless, it is likely that distortion of the airway wall at the midpoint of the preparation affected the pressure-area measurements significantly. Distortion of the airway wall as detected bronchoscopically would explain why changes in cross-sectional area were greater than changes in tracheal volume at less negative PTM values (Figs. 3–5).
Despite the influence of fixation on the ends of the airway segments, images from middle sections of our preparation demonstrated changes in the shape of the lumen consistent with changes noted in other studies. Invagination of the pars membranacea and curling of the tracheal cartilage have been described in the relaxed trachea subjected to compressive pressures(19, 35, 36). In contrast, described in these same investigations was preservation of the tracheal lumen after stimulation of the smooth muscle, even when the airway was subjected to high collapsing pressures.
Often, tracheomalacia is described bronchoscopically as“diffuse,” meaning the entire intrathoracic trachea appears abnormally collapsible, “distal,” referring to the region just proximal to the main carina, or “focal,” where there appears to be localized narrowing but the areas proximal and distal do not narrow. Clinical assessments of wall stiffness should be tailored to each situation, with careful description of what is being measured. Thus, in a child with focal collapse, it would seem reasonable to measure stiffness in the region of maximal narrowing as well as in a more normal area so that characteristics of the airway wall can be fully depicted.
We have described a technique which can differentiate true weakness of the airway wall, i.e. tracheomalacia or bronchomalacia, from airway collapse resulting from excessive compressing pressures. We speculate that, by measuring Plum through the bronchoscope suction channel, and esophageal pressure, we will be able to make similar measurements in the intact airway. This technique can yield important insights into the behavior of the central airways in health and disease.
Abbreviations
- MCh:
-
methacholine
- KBS:
-
Krebs buffer solution
- ACI:
-
airway compliance index
- Pc:
-
chamber pressure
- Plum:
-
intraluminal pressure
- PTM:
-
transmural pressure
- Caw:
-
airway compliance
References
Panitch HB, Allen JL, Alpert BA, Schidlow DV 1995 Effects of CPAP on lung mechanics in infants with acquired tracheobronchomalacia. Am J Respir Crit Care Med 150: 1341–1346.
Sotomayor JL, Godinez RI, Borden S, Wilmott RW 1986 Large-airway collapse due to acquired tracheobronchomalacia in infancy. Am J Dis Child 140: 367–371.
McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL 1989 Large airway collapse in bronchopulmonary dysplasia. J Pediatr 114: 304–307.
Baxter JD, Dunbar JS 1963 Tracheomalacia. Ann Otol Rhinol Laryngol 72: 1013–1023.
Denneny JC 1985 Bronchomalacia in the neonate. Ann Otol Rhinol Laryngol 94: 466–469.
Finder JD 1997 Primary bronchomalacia in infants and children. J Pediatr 130: 59–66.
Benjamin B, Cohen D, Glasson M 1976 Tracheomalacia in association with congenital tracheoesophageal fistula. Surgery 79: 504–508.
Benjamin B 1984 Tracheomalacia in infants and children. Ann Otol Rhinol Laryngol 93: 438–442.
Bhutani VK, Rubenstein D, Shaffer TH 1981 Pressure-induced deformation in immature airways. Pediatr Res 15: 829–832.
Bhutani VK, Shaffer TH 1982 Time-dependent tracheal deformation in fetal, neonatal, and adult rabbits. Pediatr Res 16: 830–833.
Penn RB, Wolfson MR, Shaffer TH 1988 Effect of ventilation on mechanical properties and pressure-flow relationships of immature airways. Pediatr Res 23: 519–524.
Bhutani VK, Ritchie WG, Shaffer TH 1986 Acquired tracheomegaly in very preterm neonates. Am J Dis Child 140: 449–452.
Duncan S, Eid N 1991 Tracheomalacia and bronchopulmonary dysplasia. Ann Otol Rhinol Laryngol 100: 856–858.
Mair EA, Parsons DS 1992 Pediatric tracheobronchomalacia and major airway collapse. Ann Otol Rhinol Laryngol 101: 300–309.
Miller RW, Woo P, Kellman RK, Slagle TS 1987 Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. J Pediatr 111: 779–782.
Croteau JR, Cook CD 1961 Volume-pressure and length-tension measurements in human tracheal and bronchial segments. J Appl Physiol 16: 170–172.
Burnard ED, Grattan-Smith P, Picton-Warlow CG, Grauaug A 1965 Pulmonary insufficiency in prematurity. Aust Paediatr J 1: 12–38.
Bhutani VK, Rubenstein SD, Shaffer TH 1981 Pressure-volume relationships of tracheae in fetal newborn and adult rabbits. Respir Physiol 43: 221–231.
Palombini B, Coburn RF 1972 Control of the compressibility of the canine trachea. Respir Physiol 15: 365–383.
Bhutani VK, Koslo RJ, Shaffer TH 1986 The effect of tracheal smooth muscle tone on neonatal airway collapsibility. Pediatr Res 20: 492–495.
Penn RB, Wolfson MR, Shaffer TH 1988 Effect of tracheal smooth muscle tone on collapsibility of immature airways. J Appl Physiol 65: 863–869.
Rozycki HJ, Van Houten ML, Elliott GR 1996 Quantitative assessment of intrathoracic airway collapse in infants and children with tracheobronchomalacia. Pediatr Pulmonol 21: 241–245.
Hoffstein V, Castile RG, O'Donnell CR, Glass GM, Strieder DJ, Wohl MEB, Fredberg JJ 1987 In vivo estimation of tracheal distensibility and hysteresis in normal adults. J Appl Physiol 63: 2482–2489.
Shaffer TH, Bhutani VK, Wolfson MR, Penn RB, Tran NN 1989 In vivo mechanical properties of the developing airway. Pediatr Res 25: 143–146.
Koslo RJ, Bhutani VK, Shaffer TH 1986 The role of tracheal smooth muscle contraction on neonatal tracheal mechanics. Pediatr Res 20: 1216–1220.
McFawn PK, Mitchell HW 1997 Effect of transmural pressure on preloads and collapse of immature bronchi. Eur Respir J 10: 322–329.
Vakil N, Smith W, Bourgeois K, Everbach EC, Knyrim K 1994 Endoscopic measurement of lesion size: improved accuracy with image processing. Gastrointest Endosc 40: 178–183.
Forkert L, Watanabe H, Sutherland K, Vincent S, Fisher JT 1996 Quantitative videobronchoscopy: A new technique to assess airway caliber. Am J Respir Crit Care Med 154: 1794–1803.
Jackson AC, Butler JP, Millet EJ, Hoppin FG, Dawson SV 1977 Airway geometry by analysis of acoustic pulse response measurements. J Appl Physiol Respir Environ Exercise Physiol 43: 523–536.
Hoffstein V, Zamel N, Phillipson EA 1984 Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 130: 175–178.
Brooks LJ 1990 Tracheal size and distensibility in patients with cystic fibrosis. Am Rev Respir Dis 141: 513–516.
Wittenborg MH, Gyepes MT, Crocker D 1967 Tracheal dynamics in infants with respiratory distress, stridor, and collapsing trachea. Radiology 88: 653–662.
Mitchell HW, Sparrow MP 1994 Video-imaging of lumen narrowing; muscle shortening and flow responsiveness in isolated bronchial segments of the pig. Eur Respir J 7: 1317–1325.
Olsen CR, Stevens AE, McIlroy MB 1967 Rigidity of tracheae and bronchi during muscular constriction. J Appl Physiol 23: 27–34.
Olsen CR, Stevens AE, Pride NB, Staub NC 1967 Structural basis for decreased compressibility of constricted tracheae and bronchi. J Appl Physiol 23: 35–39.
Knudson RJ, Knudson DE 1975 Effect of muscle constriction on flow-limiting collapse of isolated canine trachea. J Appl Physiol 38: 125–131.
Acknowledgements
The authors thank Robert Roache for his invaluable technical assistance, and Usha Vasthare, Ph.D., for use of the image analysis system.
Author information
Authors and Affiliations
Additional information
Supported by the Allegheny Singer Research Institute and the Helen D. Groome Beatty Trust.
Rights and permissions
About this article
Cite this article
Panitch, H., Talmaciu, I., Heckman, J. et al. Quantitative Bronchoscopic Assessment of Airway Collapsibility in Newborn Lamb Tracheae. Pediatr Res 43, 832–839 (1998). https://doi.org/10.1203/00006450-199806000-00018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199806000-00018
This article is cited by
-
The effect of bethanechol on tracheobronchomalacia in preterm infants with bronchopulmonary dysplasia: a retrospective cohort study
Journal of Perinatology (2024)
-
Tracheal compliance and limit flow rate changes in a murine model of asthma
Science in China Series C: Life Sciences (2008)