Abstract
Before weaning, arginine biosynthesis from citrulline most likely takes place in the small intestine rather than in the kidney. We studied the expression of ornithine cycle enzymes in the rat small intestine during perinatal development. The spatiotemporal patterns of expression of ornithine aminotransferase, carbamoylphosphate synthetase, ornithine transcarbamoylase, argininosuccinate synthetase, argininosuccinate lyase, and arginase mRNAs were studied by Northern blot analysis and in situ hybridization. In addition, the expression of carbamoylphosphate synthetase and argininosuccinate synthetase protein was studied by immunohistochemistry. Before birth, the developmentally more mature proximal loops of the intestine expressed the mRNAs at higher concentrations than the more distal loops. After birth, this difference was no longer obvious. The mRNAs of argininosuccinate synthetase and argininosuccinate lyase, the enzymes that metabolize citrulline to arginine, were detectable only in the upper part of the villi, whereas the other mRNAs were concentrated in the crypts. The distribution of argininosuccinate synthetase protein corresponded with that of the mRNA, whereas carbamoylphosphate synthetase protein was present in all enterocytes of the crypts and villi. Hepatic arginase mRNA could not be detected in the enterocytes. The spatial distribution of the respective mRNAs and proteins along the villus axis of the suckling small intestine indicates that the basal enterocytes synthesize citrulline, whereas the enterocytes in the upper half of the villus synthesize arginine.
Similar content being viewed by others
Main
Arginine, an intermediate metabolite of the ornithine cycle, is considered to be a dispensable amino acid for most healthy adult animals(1). Renal arginine biosynthesis, the main source of endogenous arginine in the adult(2), does not vary with fluctuations in dietary arginine intake(3–5), but depends on the vascular supply of its precursor citrulline(6). Intestinal citrulline synthesis from glutamine is, in turn, the main source of circulating citrulline(7, 8). The liver is not considered to contribute to circulating citrulline or arginine levels because of its high levels of ASS (EC 6.3.4.5), ASL (EC 4.3.2.1), and ARG (EC 3.5.3.1). In contrast to the situation in adults, arginine is regarded as a semiessential nutritional compound in many adolescent animals, including rats, because it is synthesized by the kidney at rates that are inadequate to support rapid growth(9). This discrepancy between the requirement for arginine and its endogenous biosynthetic capacity may even be aggravated in suckling rats, because the enzymes that convert citrulline to arginine in the kidney rise to adult levels only toward weaning(10, 11). Nevertheless, the finding that the ratio of the amino acid composition of rat body and milk is approximately 1 for essential amino acids, whereas it is more than 2 for arginine (and glycine)(12), clearly suggests a high degree of endogenous arginine biosynthesis during the suckling period.
The ornithine cycle enzymes except arginase are expressed in the small intestine of suckling rats(7, 10, 13, 14), but data on their cellular distribution are not yet available. By analyzing the expression and localization of ornithine-cycle enzyme mRNAs and protein by Northern blotting, in situ hybridization, and immunohistochemistry, we provide evidence that newborn rats have the capacity to synthesize citrulline and arginine at the base and the upper part of the villus of the small intestine, respectively.
METHODS
Animals. Adult Wistar rats were obtained from the HSD animal farm in Zeist (The Netherlands). Timed matings were used for the study of fetal animals. The day of copulation was taken as d 0 of pregnancy (ED 0). Birth normally occurred at the beginning of d 22 of pregnancy. The animals were weaned at 3 wk of age.
Preparation of tissue sections. Serial tissue sections were prepared exactly as described previously(15). Transverse sections in the abdominal region of prenatal animals were used, whereas of postnatal animals the proximal part of the jejunum, approximately 5 cm distal to the ligament of Treitz, was dissected free and processed further.
cRNA probes. The following cDNA fragments were cloned into the pBluescript vector to generate cRNA probes for the detection of the different ornithine cycle mRNAs: CPS (EC 6.3.4.16), the 564-bp Bam HI-SmaI fragment of the rat cDNA clone pBR-CPS5(16); OTC (EC 2.1.3.3), the 870-bp Xba I-HindIII fragment from the rat cDNA clone pOTC1(17); ASS, the 1300-bp PstI fragment from the rat cDNA clone rAS(18); ASL, the 580-bp Eco RI-HindIII fragment from the rat cDNA clone pALr-3(19); ARG, the 768-bp PstI-StuI fragment from the rat liver cDNA clone pARGr-2(20, 21); OAT (EC 2.6.1.13), the 1850-bp ecoRI fragment from the OAT cDNA clone pRLOT10(22). Probes were prepared by in vitro transcription of the appropriate DNA strand and had a specific activity of 1.5 × 109 cpm/μg. Onto each section 10μL of probe containing 40,000 cpm/μL were applied.
In situ hybridization. A comprehensive protocol to detect mRNA molecules in tissue sections using radioactively labeled cDNA probes has been described previously(15). At variance with this protocol the temperature of hybridization and washes was raised to 55°C as cRNA rather than cDNA was used.
Northern blot analysis. Total RNA was isolated as described previously(23). Ten micrograms of total RNA, denatured by heating for 10 min at 65°C in the presence of 2.2 M formaldehyde, was electrophoresed on an agarose gel containing formaldehyde. After transfer onto a nylon membrane (Amersham International plc., Little Chalfont, Buckinghamshire, UK), the RNA was hybridized to[α-32P]CTP-labeled (Amersham) cDNA probes derived from the same clones used for the cRNA labeling in the in situ hybridizations. Hybridization was performed in 50% formamide, 5 × Denhart's solution[0.1% Ficoll 400, 0.1% polyvinylpyrrolidone, 0.1% BSA (fraction V)], 0.5% SDS, and 5 × SSC (0.75 M NaCl, 75 mM Na3-citrate). cDNA probes were labeled using the random primed labeling method. After washing, the blots were exposed to phosphor screens for approximately 15 h, and signals were quantified using the PhosphorImager software (Molecular Dynamics, Sunny Vale, CA).
Immunohistochemistry. After deparaffination and rehydration, endogenous peroxidase activity in the sections was eliminated by incubation for 30 min in PBS (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.4), and 50% methanol, containing 3% (wt/vol) hydrogen peroxide. Nonspecific protein binding sites were blocked by incubation for 30 min in TENG-T buffer(10 mM Tris, 5 mM EDTA, 150 mM sodium chloride, 0.25% gelatin, 0.05% Tween 20, pH 8.0). Serial sections were incubated overnight with an appropriate dilution of rabbit polyclonal antibodies against CPS(24) and ASS(25, 26). The indirect unconjugated peroxidase-anti-peroxidase technique(27) was used to visualize binding of the primary antibodies, with 3,3-diaminobenzidine (0.5 mg/mL) as a substrate, dissolved in imidazole buffer (30 mM imidazole, 1 mM EDTA, pH 7.0), to which 0.01% hydrogen peroxide was added.
Arginase activity measurements. Arginase activity was measured by a modification of the assay described by Adlung et al.(28). Fifty milligrams of tissue were homogenized in 2 mL of a 30 mM sucrose buffer containing 5 mM MnCl2, 30 mM imidazole (pH 7.5), and 0.05% Triton X-100. This homogenate was sonicated for 30 s and activated at 55°C for 15 min. The mixture was diluted with 1 volume of H2O and centrifuged. The assay mixture (200 μL) contained 20μL of the enzyme extract, 25 mM guanido-14C-labeled L-arginine (pH 9.5, 1.0 Ci·mol-1; DuPont NEN), and 75 mM glycine (pH 9.5). The reaction was stopped after 6 min of incubation at 25°C by addition of 100 μL of 0.5 M L-arginine (pH 9.5) and 800 μL of 30% (wt/vol) phosphotungstic acid(P2O5; Malinckrodt Baker, Deventer, The Netherlands). After centrifugation, 100 μL of the supernatant were counted.
RESULTS
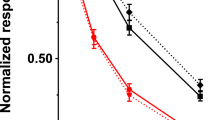
Developmental changes in mRNAs. Figure 1 shows the developmental changes of CPS, OTC, ASS, ASL, and OAT mRNA levels in the small intestine of the rat. The levels are expressed per μg of total RNA and relate to the corresponding mRNA levels in the adult liver, demonstrating that all mRNAs except ASL were highly expressed in the suckling small intestine. mRNA levels of all genes were highest during the suckling period. ASS and ASL mRNAs declined to hardly detectable levels in the second postnatal week, whereas mRNA levels of CPS and OAT declined to adult levels in the 3rd postnatal wk. The OTC mRNA level did not change with development. However, it should be acknowledged that the mRNA levels were determined perμg of total RNA, so that our data do not take into account the increase in epithelial surface as a result of the growth of the intestine. The mRNA concentration of CPS and OAT was approximately 2-fold higher in the ileum than in the jejunum, whereas concentrations of the other mRNAs studied were approximately equal in both parts of the small intestine. After birth, the horizontal gradient was either no longer detectable (OTC, ASS, and ASL), or had even become reversed, with the highest concentration in the distal (ileal) parts of the small intestine (Fig. 1, CPS and OAT).
Developmental appearance of mRNAs in the male rat jejunum (triangles) and ileum (rectangles), as determined by Northern blot analysis. The expression of OAT 1), CPS 2), OTC 3), ASS 4), and ASL 5) was measured by quantification of the hybridization signal using the PhosphorImager (Molecular Dynamics). mRNA levels are expressed as a percentage of the signal in a reference sample of adult rat liver RNA. On the x axis, the age is given in neonatal days after birth. Five animals were analyzed per age group. Values are means ± SEM.
The cellular distribution of the mRNAs pre- and postnatally was investigated using in situ hybridization. The earliest stage investigated was ED 16. Even though the small intestine is still immature at this age, the enterocytes expressed CPS, GDH, ASS, and OAT mRNAs, but not yet OTC and ASL (not shown). Two days later in development (Fig. 2), the presence of intestinal villi could be observed in the proximal loops of the small intestine, but not yet in the distal ones, in line with the craniocaudal gradient of development. The expression of CPS, OTC, ASS, and OAT mRNAs was stronger in the more mature, proximal part of the small intestine, compared with the distal part, as can be seen from the difference between loops where intestinal villi are present and loops with a simple epithelial lining of the lumen (Fig. 2). Although the morphogenesis of the villi continued toward birth, the expression of CPS, OTC, ASS, and OAT mRNAs remained essentially unchanged (shown for ASS and CPS,Fig. 3). ASL mRNA was undetectable prenatally.
Expression of the mRNAs encoding CPS (a), OTC(b), ASS (c), ASL (d), ARG (e), and OAT (f) in transverse serial sections, showing the abdominal part of an ED 18 rat fetus. The difference in development and expression of tissue-specific mRNAs between the proximal (pi) and distal(di) intestinal loops is very pronounced. Liver (li). Bar, 250 μm.
Expression of the mRNAs encoding CPS (a) and ASS (b) in transverse serial sections of an ED 20 rat fetus, showing liver (li) and proximal (pi) and distal (di) intestinal loops. Note that the expression of CPS mRNA is distributed along the entire villus, including its base, whereas ASS mRNA is absent from the villus base. ASS mRNA is expressed in the enteric neurons of the myenteric plexus (arrowhead). Only very weak hybridization was seen in the distal parts of the intestine. Bar, 100 μm.
After birth, the developmental changes in the intensity of the hybridization signals in the proximal part of the small intestine (Figs. 4 and 5), reflected that of the signal in the Northern blots, the expression of CPS, ASS, ASL, and OAT was highest at 7 d, and had clearly declined at 17 d, whereas the expression of OTC remained fairly constant. In the adult jejunum CPS, OTC, and OAT mRNAs were still expressed, but the expression of ASS and ASL mRNAs was no longer detectable, except in the neurons of the myenteric plexus (Fig. 6). GDH mRNA continued to be expressed in the adult small intestine (not shown).
Expression of CPS (a), OTC (b), ASS(c), ASL (d), ARG (e), and OAT (f) mRNAs in serial sections of the jejunum of ND 7 rats. The difference in distribution along the villus of CPS, OTC, and OAT mRNAs on the one hand and ASS and ASL mRNA on the other is very pronounced. Note the silver grains in panel e, which were found not to be due to the presence of ARG mRNA(see text). Bar, 100 μm.
Expression of CPS (a), OTC (b), ASS(c), ASL (d), ARG (e), and OAT (f) mRNAs in serial sections of the jejunum at ND 17 of rat development. Note the reduced expression of ASS and ASL mRNA compared with Figure 3. Bar, 100 μm.
Zonation of expression along the crypt-villus axis. Heterogeneity along the axis of the villus evolved parallel with villus development. At ED 18 the expression of CPS, OTC, and OAT mRNAs was seen in the epithelium along the entire villus (Fig. 2), whereas ASS levels were weaker at the intervillus region than at the upper part of the villi. At ED 20 the concentration of CPS, OTC, and OAT mRNA was higher at the intervillus region, whereas ASS mRNA was absent from this part of the epithelium (shown for CPS and ASS, Fig. 3). After birth, this difference became more pronounced: ASS and ASL were detectable only in the upper part of the villi, whereas CPS, OTC, and OAT were present mainly in the basal part of the villi and in the developing crypts (Figs. 3–5).
Antibodies against two key enzymes, CPS and ASS, were applied to investigate to what extent mRNA and protein colocalize (Fig. 7). Whereas CPS mRNA was concentrated at the basal part of the villi and in the developing crypts, CPS protein was present in all enterocytes. ASS protein, on the other hand, colocalized with ASS mRNA, and was confined to the upper part of the villi. ASS protein was, except for the enteric neurons, no longer detectable in the adult intestine, whereas CPS protein remained present.
Presence of CPS (a, c, e, g, and i) and ASS (b, d, f, h, and j) protein in the rat jejunum of ED 20 (a and b), ND 1 (c and d), ND 8(e and f), ND 18 (g and h), and adult(i and j). ASS protein is present only at the upper half of the villi, whereas CPS protein is seen in the developing crypts and the enterocytes along the entire villus. In the adult jejunum, ASS protein is only detected in the enteric neurons (arrowheads). Bar, 100 μm.
Arginase. To demonstrate that the small intestine of the suckling rat indeed has the capacity to synthesize arginine, arginase activity should be absent. Arginase mRNA was undetectable at all ages investigated, both by in situ hybridization and Northern blot analysis. The only exception was a highly reproducible in situ hybridization signal at ND 7 (Fig. 4e). This signal could not be eliminated by RNAase treatment of the section before hybridization (not shown). Moreover, arginase mRNA was not detectable on Northern blots (Fig. 8a) and arginase activity was virtually undetectable in jejunal extracts before ND 18 (Fig. 8b). Therefore, the in situ hybridization signal observed at ND 7 was judged to be nonspecific. After ND 18, arginase activity in the jejunum showed an abrupt increase. The adult values were found to be 8.7 ± 0.5% (n = 5) of the activity measured in the adult rat liver.
Arginase in the jejunum. (a) Northern blot analysis shows the absence of hepatic arginase mRNA in rat jejunum. The blot is representative of five separate experiments, run in parallel with those shown in Figure 1. (b) Development of arginase activity in homogenates of rat jejunum. On the x axis the age of rats is given, on the y axis the enzyme activity in nanomoles/min/mg of protein. Values are means ± SEM; n = 5 animals per age group.
DISCUSSION
The metabolic demands and capacities of organs can change during development. An intriguing example is found in the small intestine. In adult mammals, the intestine produces citrulline from glutamine. After release of citrulline into the circulation, the kidney converts citrulline into arginine. However, the newborn kidney acquires the capacity to synthesize arginine from citrulline only gradually, with adult levels being established around weaning(11, 29). Before weaning, arginine biosynthesis most likely takes place in the gut rather than the kidney(10), because the activities of the enzymes involved in arginine biosynthesis are high, and those that use arginine or its precursors are low.
In the small intestine of mice, rats, and pigs, the activities of the enzymes involved in citrulline biosynthesis (CPS, N-acetylglutamate synthetase, and OTC), as well as those involved in the conversion of citrulline into arginine (ASS and ASL) peak shortly after birth and gradually decline to adult levels at weaning(10, 30–33). Similarly, enzymes that convert glutamine to ornithine (phosphate-dependent glutaminase, pyrroline-5-carboxylate synthase, and OAT all show activities well above adult levels in the period before weaning(13, 14, 31, 33–36). Because neonatal transgenic mice, in which the OAT gene was disrupted, have very low circulating arginine levels, it is plausible that the activity of OAT in the small intestine is directed toward the synthesis of ornithine in the neonatal period(37). On the other hand, the activity of pyrroline-5-carboxylate reductase, the enzyme that can divert pyrroline-5-carboxylate toward proline, is low during the suckling period in rat and hamster(35, 38). Furthermore, ornithine decarboxylase activity, which diverts ornithine toward polyamine biosynthesis, is very low during the suckling period, but shows a sharp, transient burst in activity at the onset of weaning(14, 39). Finally, we and others(10, 14, 38, 40) found that intestinal arginase activity is virtually absent in the intestine until it appears abruptly weaning.
The absence of a hybridization signal with our ARG probe in gut tissue at a time when arginase activity is easily measurable, i.e. after weaning, shows that this arginase activity arises from an arginase isoform that is different from that in liver(41–43). Indeed, an immunologically distinct arginase in rat mammary gland and kidney has been isolated(44), and cloned(45, 46). The cellular distribution of this nonhepatic arginase gene product in the gut remains to be established.
The ASS mRNA and protein in the neurons of the myenteric plexus of the adult small intestine colocalizes with neuronal nitric oxide synthase mRNA(our unpublished results), suggesting the existence of a neuronal arginine-citrulline cycle for regeneration of arginine for nitric oxide synthesis. Such a cycle has been described in several other nitric oxide-producing cell types, such as endothelial cells(47, 48) and macrophages(49).
Perhaps the most intriguing aspect of the present study is why the mRNAs for glutaminase(13, 50), CPS, OTC, and OAT are concentrated at the base of the villi and in the developing crypts, whereas ASS and ASL can be demonstrated only in the upper part of the villi. This difference in distribution can be detected as early as ED 18, is easily visible at ED 20, and persists until weaning. The question arises in what respect the enterocytes occupying the upper half of the villus, in the fetal and neonatal intestine, differ from those occupying the same position after weaning. In this respect, it might be of relevance that dramatic maturational changes occur in the rat small intestine in the 3rd postnatal wk, including an acceleration of the turnover rate of enterocytes(51). It is tempting to relate the disappearance of ASS and ASL gene expression from the enterocytes in the 3rd wk to this higher turnover rate of the enterocytes. ASS and ASL can apparently be expressed only in the enterocytes with a long lifetime. Another conspicuous property of suckling enterocytes is the presence of a giant supranuclear lysosome. These highly typical cells disappear at weaning and, prematurely, upon treatment with glucocorticoids or polyamines.
Metabolic zonation, that is, the spatial separation of the metabolic pathways among otherwise similar cells, potentially exists in two directions of the gut. The first direction, i.e. heterogeneity of the enterocytes along the proximal-distal axis of the organ, is pronounced during prenatal development, but is less obvious postnatally. The zonation in the second direction, i.e. along the crypt-villus axis, appears to be a characteristic of the citrulline/arginine biosynthetic pathway. In view of the fact that CPS, OTC, ASS, ASL, and OAT should all be present within one enterocyte to sustain arginine biosynthesis, it may seem surprising, at first glance, that ASS and ASL mRNA and protein are absent from the enterocytes in the crypts and at the base of the villi. This finding implies that the“young” enterocytes, at the base of the villus, can synthesize only citrulline, whereas those near the top of the villus cannot only synthesize citrulline, but can also convert it into arginine. Such a two-compartment system along the villus axis may enable the gut to simultaneously meet the needs for the synthesis of citrulline and arginine during the suckling period. This metabolic zonation is reminiscent of that in hepatocytes along the portocentral radius of the liver lobule (the smallest metabolic unit of that organ)(52–54).
We hypothesize that the capacity to produce arginine in the small intestine of sucklings is beneficial for several reasons. First, during this developmental period, the intestine has a very high growth rate and, thus, a high demand for amino acids. We already cited the study by Davis et al.(12), implying a high net synthesis of arginine during the suckling period. Second, the surface area of the intestinal epithelium has a high potential not only for digestion and absorption of nutrients, but also for damage by noxious dietary substances and micro-organisms. To deal with the latter, the intestinal mucosa contains both physical barriers and immunologic defenses. During development, the need for a mucosal means of defense arises abruptly at birth. However, the specific mucosal immune system matures during suckling and becomes fully functional only after weaning(55). Arginine has been identified as a potential immunomodulatory substance(56), partly because it is a substrate for nitric oxide synthase. Adult enterocytes have been shown to express this enzyme(57, 58), but a relation with a local intestinal arginine production in the neonate remains to be established.
Abbreviations
- ARG:
-
arginase
- ASL:
-
argininosuccinate lyase
- ASS:
-
argininosuccinate synthetase
- CPS:
-
carbamoylphosphate synthetase
- OAT:
-
ornithine aminotransferase
- OTC:
-
ornithine transcarbamoylase
- ED:
-
embryonal day
- ND:
-
neonatal day
References
Visek WJ 1986 Arginine needs, physiological state and usual diets. A reevaluation. J Nutr 116: 36–46
Featherston WR, Rogers QR, Freedland RA 1973 Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 224: 127–129
Dhanakoti SN, Brosnan JT, Brosnan ME, Herzberg GR 1992 Net renal arginine flux in rats is not affected by dietary arginine or dietary protein intake. J Nutr 122: 1127–1134
Hartman WJ, Prior RL 1992 Dietary arginine deficiency alters flux of glutamine and urea cycle intermediates across the portal-drained viscera and liver of rats. J Nutr 122: 1472–1482
Castillo L, Ajami AM, Branch S, Chapman TE, Yu Y-M, Burke JF, Young VR 1994 Plasma arginine kinetics in adult man: response to an arginine-free diet. Metabolism 43: 114–122
Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME 1990 Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol 259:E437–E442
Windmüller HG 1982 Glutamine utilization by the small intestine. Adv Enzymol 53: 201–237
Hoogenraad N, Totino N, Elmer H, Wraight C, Alewood P, Jones RB 1985 Inhibition of intestinal citrulline synthesis causes severe growth retardation in rats. Am J Physiol 249:G792–G799
Milner JA, Wakeling AE, Visek WJ 1974 Effect of arginine deficiency on growth and intermediary metabolism in rats. J Nutr 104: 1681–1689
Hurwitz R, Kretchmer N 1986 Development of arginine-synthesizing enzymes in mouse intestine. Am J Physiol 251:G103–G110
Morris SM, Sweeney WE, Kepka DM, O'Brien WE, Avner ED 1991 Localization of arginine biosynthetic enzymes in renal proximal tubules and abundance of mRNA during development. Pediatr Res 29: 151–154
Davis TA, Fiorotto ML, Reeds PJ 1993 Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr 123: 947–956
Shenoy V, Roig JC, Kubilis P, Neu J 1996 Characterization of glutaminase in the developing rat small intestine. J Nutr 126: 1121S–1130S
Riby JE, Hurwitz RE, Kretchmer N 1990 Development of ornithine metabolism in the mouse intestine. Pediatr Res 28: 261–265
Moorman AFM, de Boer PAJ, Vermeulen JLM, Lamers WH 1993 Practical aspects of radio-isotopic in situ hybridization on RNA. Histochem J 25: 251–260
de Groot CJ, Zonneveld D, de Laaf RTM, Dingemanse MA, Mooren PG, Moorman AFM, Lamers WH, Charles R 1986 Developmental and hormonal regulation of carbamoylphosphate synthetase gene expression in rat liver: evidence for control mechanisms at different levels in the perinatal period. Biochim Biophys Acta 866: 61–67
Takiguchi M, Miura S, Mori M, Tatibana M, Nagata S, Kaziro Y 1984 Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci USA 81: 7412–7416
Morris MS 1988 Nucleotide sequence of the cDNA encoding the rat argininosuccinate synthetase, Nucleic Acids R. es 16: 9352
Amaya Y, Matsubasa T, Takiguchi M, Kobayashi K, Saheki T, Kawamoto S, Mori M 1988 Amino acid sequence of rat argininosuccinate lyase deduced from cDNA. J Biochem 103: 177–181
Kawamoto S, Amaya Y, Oda T, Kuzumi T, Saheki T, Kimura S, Mori M 1986 Cloning and expression in Escherichia coli of cDNA for arginase of rat liver. Biochem Biophys Res Commun 136: 955–961
Kawamoto S, Amaya Y, Murakami K, Tokunaga F, Iwanaga S, Kobayashi K, Saheki T, Kimura S, Mori M 1987 Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver arginase. J Biol Chem 262: 6280–6283
Mueckler MM, Pitot HC 1985 Sequence of the precursor to rat ornithine aminotransferase deduced from a cDNA clone. J Biol Chem 260: 12993–12997
Chomszynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
Charles R, de Graaf A, Moorman AFM 1980 Radioimmunochemical determination of carbamoylphosphate synthetase (ammonia) content of adult rat liver. Biochim Biophys Acta 629: 36–49
Schmidlin A, Kalbacher H, Wiesinger H 1997 Presence of argininosuccinate synthetase in glial cells as revealed by peptide-specific antisera. Biol Chem 378: 47–50
Nakamura H, Saheki T, Nakagawa S 1990 Differential cellular localization of enzymes of L-arginine metabolism in the rat brain. Brain Res 530: 108–112
Sternberger LA, Hardy PH, Cululis JJ, Meyer HG 1970 The unlabelled antibody enzyme method of immunohistochemistry. J Histochem Cytochem 20: 315–333
Adlung J, Lorentz K, Grazikowske H 1971 Ueber eine neue, einfache radiochemische Bestimmung von Arginase. Z Klin Chem Klin Biochem 9: 411–414
Wu G, Knabe DA 1995 Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol 269: 621–629
Ryall JC, Quantz MA, Shore GC 1986 Rat liver and intestinal mucosa differ in the developmental pattern and hormonal regulation of carbamoylphosphate synthetase I and ornithine carbamoyl transferase gene expression. Eur J Biochem 156: 453–458
Yamada E, Wakabayashi Y 1991 Development of pyrroline-5-carboxylate synthase and N-acetylglutamate synthase and their changes in lactation and aging. Arch Biochem Biophys 291: 15–23
Dubois N, Cavard C, Chasse JF, Kamoun P, Briand P 1988 Compared expression levels of ornithine transcarbamylase and carbamoylphosphate synthetase in liver and small intestine of normal and mutant mice. Biochim Biophys Acta 950: 321–328
Wu G, Knabe DA, Flynn NE 1994 Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299: 115–121
Nagy LE, Kretchmer N 1988 Utilization of glutamine in the developing rat jejunum. J Neurochem 118: 189–193
Schiller CM, Southern JT, Walden R 1981 Glutamine and glutamate utilization in the hamster small intestine. J Appl Biochem 3: 147–156
Remesar X, Arola L, Palou A, Alemany M 1985 Activities of amino acid metabolizing enzymes in the stomach and small intestine of developing rats. Reprod Nutr Dev 25: 861–866
Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D 1995 Mice lacking ornithine-d-aminotransferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat Genet 11: 185–190
Herzfeld A, Raper SM 1976 Enzymes of ornithine metabolism in adult and developing rat intestine. Biochim Biophys Acta 428: 600–610
Luk GD, Marton LJ, Baylin SB 1980 Ornithine decarboxylase is important in intestinal mucosal maturation and recovery from injury in rats. Science 210: 195–198
Blachier F, M'Rabet-Touil H, Posho L, Darcy-Vrillon B, Duée P-H 1993 Intestinal arginine metabolism during development. Evidence for de novo synthesis of L-arginine in newborn pig enterocytes. Eur J Biochem 216: 109–117
Herzfeld A, Raper SM 1976 The heterogeneity of arginases in rat tissues. Biochem J 153: 469–478
M'Rabet-Touil H, Lerminiaux H, Duée PH, Blachier F 1996 Evidence for increased anionic arginase activity in pig enterocytes during development. Biochem Mol Biol Int 38: 197–204
Glass RD, Knox WE 1973 Arginase isozymes of rat mammary gland, liver, and other tissues. J Biol Chem 248: 5785–5789
Jenkinson CP, Grigor MR 1994 Rat mammary arginase: Isolation and characterization. Biochem Med Metabol Biol 51: 156–165
Gotoh T, Araki M, Mori M 1997 Chromosomal localization of the human arginase II gene and tissue distribution of its mRNA. Biochem Biophys Res Commun 233: 487–491
Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD 1996 Cloning and characterization of the human type II arginase gene. Genomics 38: 118–123
Hecker M, Sessa WC, Harris HJ, Änggård EE, Vane JR 1990 The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci USA 87: 8612–8616
Hattori Y, Campbell EB, Gross SS 1994 Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. J Biol Chem 269: 9405–9408
Wu G, Brosnan JT 1992 Macrophages can convert citrulline into arginine. Biochem J 281: 45–48
Nagy LE, Pittler A, Kretchmer N 1988 Development of glutaminase along the villus-crypt axis in the jejunum of rat. J Pediatr Gastr Nutr 7: 907–913
D'Harlingue AE, Kwong LK, Morrill JS, Sunshine P, Tsuboi KK 1986 Growth and differentiative maturation of the rat enterocyte. J Pediatr Gastr Nutr 5: 956–963
Meijer AJ, Lamers WH, Chamuleau RAFM 1990 Nitrogen metabolism and ornithine cycle function. Physiol Rev 70: 701–748
Häussinger D, Lamers WH, Moorman AFM 1992 Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme 46: 72–93
Jungermann K, Katz N 1989 Functional specialization of different hepatocyte populations. Physiol Rev 69: 708–764
Brandtzaeg P, Nilssen DE, Rognum TO, Thrane PS 1991 Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am 20: 397–471
Barbul A 1990 Arginine and immune function. Nutrition 6: 53–58
Cattell V, Jansen A 1995 Inducible nitric oxide synthase in inflammation. Histochem J 27: 777–784
M'Rabet-Touil H, Blachier F, Morel M, Darcy-Vrillon B, Duée PH 1993 Characterization and ontogenesis of nitric oxide synthase activity in pig enterocytes. FEBS Lett 331: 243–247
Acknowledgements
The authors thank C. Gravemeijer and C. Hersbach for photographical assistance. Dr. David Valle (Howard Hughes Medical Institute, Baltimore, MD), Dr. M. Mori (Kuwamoto University School of Medicine, Kuwamoto, Japan), and Dr. S. M. Morris (Howard Hughes Medical Institute, Baylor College, Houston, TX) are acknowledged for making cDNAs available for this study. Dr H. Wiesinger (University of Tübingen, Germany) and Dr. T. Saheki (Kagoshima University, Japan) are gratefully acknowledged for their gift of antibodies.
Author information
Authors and Affiliations
Additional information
Supported in part by the Dutch Organisation for Scientific Research (NWO), Grants 902-23-098 (W.d.J.) and 900-523-155 (M.A.D.).
Rights and permissions
About this article
Cite this article
de Jonge, W., Dingemanse, M., de Boer, P. et al. Arginine-Metabolizing Enzymes in the Developing Rat Small Intestine. Pediatr Res 43, 442–451 (1998). https://doi.org/10.1203/00006450-199804000-00002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199804000-00002