Keeping abreast of new research into the many and complex risk factors underpinning development of psychotic symptoms is essential when considering primary prevention, early detection and treatment of psychosis. In this three-part review, our aim is to outline the current understanding of key risk factors for psychotic symptom development in relation to a timeline (Fig. 1). In part 1 (Romain Reference Romain, Eriksson and Onyon2019a) we discussed early-life risk factors and in part 3 (Romain Reference Romain, Eriksson and Onyon2019b) we will examine the final common pathways to psychosis and possible preventative strategies which may help to ameliorate the risks. Overall, this series aims to update knowledge of risk factors for psychosis and provide a basis for discussion focused on making positive changes for the future.
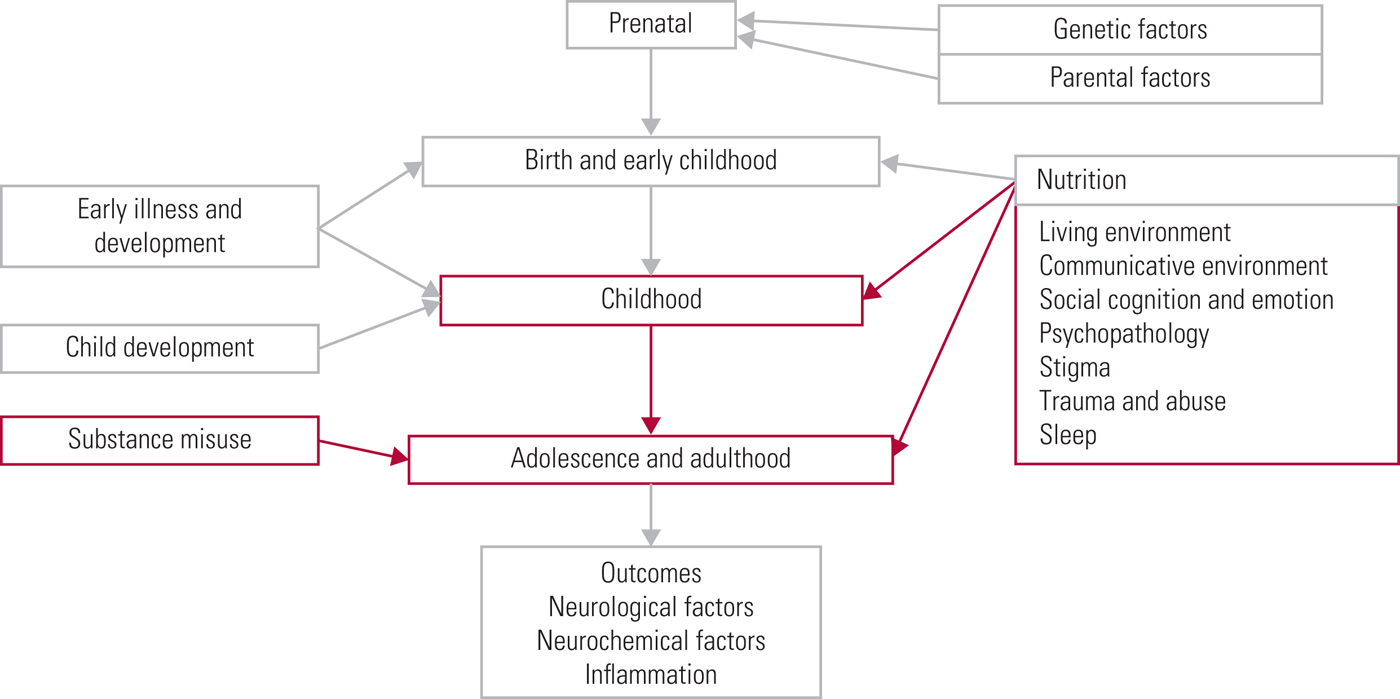
Fig 1 The psychosis risk timeline: factors over the lifespan that can affect the risk for psychosis.
The present article covers the second part of the psychosis risk timeline and therefore focuses on the risk factors that would have their greatest impact on individuals during their later childhood, adolescence and adulthood. To ensure a comprehensive review is made of current research, we have included not only studies that demonstrate a direct link between risk factors and psychosis itself, but also studies that examine risk factors linked with being categorised as ‘at high risk of developing psychosis’ (which implies that only a proportion will make the transition to psychotic illness). Every effort has been made to make clear exactly what link and evidence is discussed in each study, to allow the reader to form a nuanced and accurate opinion. We recognise that psychosis is a broad concept and that a limiting factor for any endeavour to comprehensively collect and analyse data on the topic is made more difficult by the range of definitions used in current research. Data are discussed here that pertain to the umbrella term of psychosis, but it is recognised that within this concept may fall a variety of terms, including schizophrenia, schizophrenia spectrum disorders and non-affective psychosis.
Social environment and psychology
Living environment
Urban setting
Various environmental factors contribute to the relative risk of psychosis. Data consistently suggest that the risk of schizophrenia is lower in rural areas (van der Werf Reference van der Werf, Hanssen and Köhler2012; McKenzie Reference McKenzie, Murray and Booth2013; Laurens Reference Laurens, Luo and Matheson2015) and that children living in urban environments are more likely to experience psychotic symptoms (the odds ratio (OR) is 1.76 at age 12). Suggested mediating factors include lower levels of social cohesion and higher levels of crime victimisation (Newbury Reference Newbury, Arseneault and Caspi2016). Other proposed factors include changes in access to outdoor green space, the actual and perceived quality of the environment, and higher background noise levels in urban settings (McKenzie Reference McKenzie, Murray and Booth2013; Savale Reference Savale2014). Another candidate factor is pollution, which has been suggested to cause neurological changes via inflammation of nervous tissue, disruption of the blood–brain barrier and altered ratios of neurotransmitters such as glutamate and gamma-aminobutyric acid (GABA) (Attademo Reference Attademo, Bernardini and Garinella2017).
Socioeconomic status
Systematic reviews have corroborated the effect of low socioeconomic status, taking into account access to resources, social influence and parental education as risk factors for schizophrenia spectrum disorders (Laurens Reference Laurens, Luo and Matheson2015). Suggested mediators carrying this effect include stressful life events, lacking social support, isolation and the perception of reduced control (Hur Reference Hur, Choi and Yun2015). Evidence suggests that low parental socioeconomic status may have a greater impact on symptom progression than on symptom onset (Hur Reference Hur, Choi and Yun2015; Laurens Reference Laurens, Luo and Matheson2015).
Increased geographical (residential) mobility in the early years of life has been shown to increase the risk for psychotic disorders. This risk increased with age at moving and with number of moves, with the relative risk for schizophrenia being greatest for those who moved three or more times at age 14 (RR = 4.80). Mobility during adolescence is thought to disrupt social environments and psychosocial development. However, increased mobility could be a marker for other adversities, and there is the possibility that individuals with prodromal symptoms change home more frequently (Paksarian Reference Paksarian, Eaton and Mortensen2015).
Migration and refugee status
Migration has been repeatedly linked with an increase in the risk of psychosis. Tarricone et al (Reference Tarricone, Boydell and Kokona2016) demonstrated incidence rate ratios (IRR) of 1.93 for internal migrants and 1.79 for external migrants compared with controls. Suggested mechanisms for this association include the increased stress of adapting to a new social environment and the experience of prejudice or discrimination, as well as the stress inherent in migration itself (Kirkbride Reference Kirkbride and Hollander2015; Tarricone Reference Tarricone, Boydell and Kokona2016). Tarricone et al (Reference Tarricone, Boydell and Kokona2016) demonstrated in migrants higher levels of being single/not in a relationship, living alone and cannabis use, which could in themselves contribute to increased risk.
Akdeniz et al (Reference Akdeniz, Schäfer and Streit2017) noted that the IRR for migrants versus non-migrants ranged from 2:1 to 5:1 and that risk was higher in both first- and second-generation migrants. They examined the impacts of migration and environmental factors on perigenual anterior cingulate cortex (pACC) volume in second-generation migrants, as the pACC has been suggested as showing functional alterations in response to social stress. Their magnetic resonance imaging (MRI) data demonstrated a decrease in grey matter volume of the pACC in male second-generation migrants. An association was found between decreased ACC grey matter volume and early-life urban upbringing. Overall, conclusions suggested that grey matter changes were mediated by chronic social stress.
Refugees have an even higher risk of psychosis. A large study showed that refugees were at increased risk of non-affective psychosis compared with both the native population (adjusted hazard ratio 2.9) and non-refugee migrants (adjusted hazard ratio 1.7) (Hollander Reference Hollander, Dal and Lewis2016). This hypothesis was corroborated further in a systematic review in which an increased risk of psychotic disorders was again found in refugees compared with both the indigenous population and non-refugee migrants. This elevated risk was more pronounced in refugee men (Dapunt Reference Dapunt, Kluge and Heinz2017).
Communicative environment
Family functioning
Hameed & Lewis (Reference Hameed and Lewis2016) combined data on family functioning from five studies in a systematic review. Mothers with schizophrenia were demonstrated, on observation, to be less responsive and show less proximity to their infants. Less affectionate involvement from the mother, anxious attachment by the children and absent stranger anxiety in the first year of life have each been more frequently demonstrated. Family units in some studies have been described as more disorganised and unpredictable. Poor social adjustment at age 5 predicted later psychosis development (OR = 4.5). They further reported that adults with more negative symptoms were more likely to have been described by teachers as isolated and passive during school years, with those with more predominant positive symptoms more likely to have been described as overactive and distractible. Other significant predictors from childhood included attention, memory and motor skills assessed at age 9 (ORs of 20, 25 and 20 respectively) and school-age emotional symptoms (OR = 2.9).
Parent–child communication
Poor parent–child relationships have been consistently demonstrated to be linked to an increased risk of schizophrenia spectrum disorders (Laurens Reference Laurens, Luo and Matheson2015). Parental communication deviance (the use of vague, fragmented or contradictory language) has been investigated as a form of poor communication with children. A meta-analysis by de Sousa et al (Reference de Sousa, Varese and Sellwood2014) found communication deviance to be highly prevalent in the parents of individuals with psychotic illnesses and it has been associated with development of psychosis in offspring. The nature of the link has been discussed in the literature, but there is no conclusive evidence to demonstrate causality or reverse causality. Another theory suggests a shared genetic vulnerability for communication deviance and psychosis (de Sousa Reference de Sousa, Varese and Sellwood2014).
Social defeat
Social defeat, i.e. prolonged exposure to social exclusion or adversity, has been proposed as a possible risk factor for psychosis. Valmaggia et al (Reference Valmaggia, Day and Garety2015a) studied the relationship between social defeat and paranoid appraisal in an experimental social environment. Study participants at clinical high risk of psychosis (Box 1) reported higher baseline levels of social defeat than controls. In those at high risk, a history of social defeat was associated with a significantly increased chance of making paranoid appraisals of social interactions. Those at high risk further reported increased loneliness and poorer quality relationships. Those reporting a poorer quality of relationships reported higher symptom severity and lower overall functioning. This is important, as it is thought that good social support reduces the negative effect of stressful life events (Robustelli Reference Robustelli, Newberry and Whisman2017). However, the direction of this association is not clear, as the case group here comprised high-risk participants, rather than those with established psychosis.
Box 1 The clinical high-risk state
In the research literature, study participants who meet certain well-established prodromal criteria are described as being at clinical high-risk for psychosis. The criteria include the at-risk mental state (ARMS), the prodromal period and the ultra-high-risk state (UHR).
UHR criteria include one or more of the following, together with functional decline: attenuated psychotic symptoms; brief intermittent psychotic symptoms; a family history of psychosis in a first-degree relative.
(Fusar-Poli Reference Fusar-Poli, Borgwardt and Bechdolf2013)Social cognition and emotion
Theory of mind (TOM) deficit
Social cognition, and particularly theory of mind (TOM) deficit, is a well-known feature of schizophrenia and has been implicated as a marker of vulnerability for its development. One review and meta-analysis concluded that TOM impairments are demonstrated in both chronic and first-episode psychosis. Most interestingly, performance is also impaired, although to a lesser extent, in the clinical high-risk group and in unaffected first-degree relatives compared with controls (Bora Reference Bora and Pantelis2013).
A systematic review by Pickup (Reference Pickup2008) concluded that the TOM deficit in people with schizophrenia is independent of executive function impairment, implying separate underlying dysfunctions. More recently, TOM has been discussed as an endophenotype in genetic association studies. Links have been demonstrated with both single nucleotide polymorphisms and specific copy number variations, underpinning genetically the link between schizophrenia and TOM (Martin Reference Martin, Robinson and Dzafic2014).
Affect recognition
Poor affect recognition has been associated with psychosis. Addington et al (Reference Addington, Piskulic and Perkins2012) were able to demonstrate that this deficit was present in both their clinical high-risk group and help-seeking controls in comparison with a non-psychiatric control group. However, it was not shown in this work to be a marker for psychosis development. Further evidence from work by Allott et al (Reference Allott, Schäfer and Thompson2014) does suggest that some specific alterations in emotion recognition may predict transition to a psychotic disorder. Their 12-month follow-up study of high-risk young people reported that those who transitioned to a psychotic disorder showed, at baseline, more difficulty in interpreting neutral emotion and better identification of fear. At baseline, the group that subsequently transitioned were more likely to mislabel neutral emotion as fear (8.5 v. 1.8%). Studies of functional MRIs in patients with schizophrenia have demonstrated increased activation of the hippocampus, parahippocampus, amygdala, thalamus and cuneus – classic facial expression processing regions – when viewing a neutral face. This suggests that there may be a neurological link underpinning the altered facial expression recognition findings (Allott Reference Allott, Schäfer and Thompson2014).
Thought disorder
When working to improve psychosis risk prediction tools, Perkins et al (Reference Perkins, Jeffries and Cornblatt2015) identified difficulty with concentration, suspiciousness and severity of unusual thought content as good predictors of transition to psychosis. Reduced ideational richness, which they defined this as ‘poor performance in areas such as abstract thinking, considering alternative positions, and following everyday conversations’, was a further important factor (Perkins Reference Perkins, Jeffries and Cornblatt2015). Coping ability, self-efficacy and control beliefs have also been found to be impaired in both at-risk individuals and in those who have had their first psychotic episode (Schmidt Reference Schmidt, Grunert and Schimmelmann2014). During the at-risk period coping problems resembled those seen in people with depression, with low self-concept reported by 48% of the at-risk sample compared with 18% of the first-episode psychosis sample. On the other hand, in the first psychotic episode, difficulties were related to being overly self-confident.
Motivational impairments
The clinical high-risk population has been shown to experience significant impairments in motivation, which can lead to withdrawal and isolation. Isolation in itself may lead to difficulties in coping with stressful events. Motivation changes, therefore, could be another precursor to the later development of negative symptoms, although again this link is discussed not in the context of those with diagnosable illness, but rather in those at high risk of psychosis (Schlosser Reference Schlosser, Fisher and Gard2014).
Psychopathology
Depression and anxiety disorders
Those categorised as being at ultra-high risk of developing psychosis have been found to have higher rates of psychiatric comorbidity, associated with poorer clinical outcomes. The most common comorbid diagnoses are depression and anxiety disorders. In one study, 50.3% had at least one comorbid diagnosis (Lim Reference Lim, Rekhi and Rapisarda2015). Those with comorbidity also had more severe symptoms, higher distress and lower functioning. However, presence of comorbidity did not appear to influence transition to psychosis, suggesting that, although associated, non-psychotic comorbidity might not be an independent risk factor. Alternatively, a larger burden of overlapping risk factors might be present in patients with both psychotic and non-psychotic illness, explaining the link with more severe disease. Depression and anxiety can form part of the psychosis prodrome, potentially obfuscating possible causation (Lim Reference Lim, Rekhi and Rapisarda2015).
Individuals at clinical high-risk of psychosis have been found to have altered personality profiles, with certain personality traits being protective and others increasing vulnerability (Song Reference Song, Kang and Kim2013). For example, they demonstrated higher harm avoidance as well as lower reward dependence than controls. They further demonstrated lower self-directedness and cooperativeness. Similar findings were reported for those experiencing a first episode of psychosis. Baseline low cooperativeness was described as a predictive factor for conversion to psychosis.
Behavioural problems
A link has been suggested between deviant behaviours and psychopathology in childhood and risk for schizophrenia spectrum disorders. A systematic review found that, in early adolescence, risk for schizophrenia spectrum disorders was increased not only in individuals with certain psychopathologies, such as depression, conduct disorder and anxiety, but also in those with behavioural problems (Laurens Reference Laurens, Luo and Matheson2015). Another systematic review and meta-analysis concluded that cognitive deficits are present in adolescents at either clinical (d = 0.34–0.71) or familial (d = 0.24–0.81) high risk of psychosis (Bora Reference Bora, Lin and Wood2014). Furthermore, co-occurrence of genetic risk and attenuated psychotic symptoms was associated with more severe cognitive dysfunction. Transition to psychosis was associated with more severe cognitive deficits (d = 0.31–0.49).
Impaired cognitive functioning
A further meta-analysis of cognitive function in individuals at clinical high risk of psychosis found significant impairments in neurocognitive functioning (Hedges' g = −0.344) (Fusar-Poli Reference Fusar-Poli, Deste and Smieskova2012). Vulnerability to psychosis was associated with deficits in executive function, verbal fluency, attention, visual and verbal memory as well as working memory. Scores on tests of general intelligence were lower in individuals who transitioned to psychosis (within 19 months) than in those who did not. They also showed poorer verbal fluency as well as poorer verbal, visual and working memory compared with those who did not transition. There were notable differences between males and females, with females demonstrating better cognitive performance. This may be related to the often later onset of schizophrenia in females (Fusar-Poli Reference Fusar-Poli, Deste and Smieskova2012). Interestingly, some evidence suggests above-average performance, for example, general academic performance at age 20, in healthy relatives of individuals with psychosis compared with the general population (Karlsson Reference Karlsson2001).
Several recent studies have looked into the value of automated speech analysis. This involves finding speech features that, in groups of high-risk patients, were able to help predict onset of psychosis. Bedi et al (Reference Bedi, Carrillo and Cecchi2015), in their proof-of-concept study of 34 high-risk young people, were able to predict the transition of 5 participants with 100% accuracy. Corcoran et al (Reference Corcoran, Carrillo and Fernández-Slezak2018) demonstrated 79–83% accuracy in predicting transition in their clinical high-risk group. Both sets of authors comment that this avenue of research is pursued with the hope of developing objective clinical tests to more accurately predict transition to psychosis. This would allow for interventions to be better targeted. However, it is worth considering the implications for the patients tested in terms of possible psychological distress, as this itself might affect their risk of transitioning (Bedi Reference Bedi, Carrillo and Cecchi2015; Corcoran Reference Corcoran, Carrillo and Fernández-Slezak2018).
Stigma
Evidence suggests that both stigma related to the label of high risk and stigma related to symptoms of psychosis affect patients' experiences of their illness. Feelings of shame related to labelling and symptoms of psychosis have been shown to be associated with increased anxiety and depression (Yang Reference Yang, Link and Ben-David2015). Psychotic symptoms can be a source of stress in themselves. Rapado-Castro et al (Reference Rapado-Castro, McGorry and Yung2015) demonstrated that greater distress associated with attenuated psychotic symptoms, anxiety and substance use is linked with a greater chance of transition to psychosis (with hazard ratios of 1.77, 1.59 and 3.8 respectively). The most intense source of distress in their clinical high-risk population was social and functional difficulty (78.1%), followed by depressive symptoms (58.9%) and attenuated positive symptoms (58.5%).
Trauma and abuse
Sexual, physical and emotional abuse
Recent analysis demonstrated that, in a youth population, those who reported sexual abuse were at a ten times greater risk of later being given a diagnosis of a psychotic disorder (Bourgeois Reference Bourgeois, Lecomte and Daigneault2018). The authors pointed out that the limitations of this research include many studies being based on retrospective reports, which may be influenced by the abnormal mental state created by the psychosis itself (Bebbington Reference Bebbington2018). Meta-analysis has demonstrated that childhood adversity is strongly associated with an increased risk of psychosis, with an odds ratio of 2.8 (Varese Reference Varese, Smeets and Drukker2012). The authors extrapolated from their data that, if childhood adversity (in the form of, for example, sexual, physical or emotional abuse) was eliminated, assuming causality, the number of people with psychosis would be reduced by 33%.
Shevlin et al (Reference Shevlin, Houston and Dorahy2008) compared results from the National Comorbidity Survey (NCS) and British Psychiatric Morbidity Survey (BPMS). The NCS reported that molestation and childhood physical abuse were associated with odds ratios for psychosis of 2.51 and 4.20 respectively. In comparison, in the BPMS sample the odds ratio for sexual abuse was 5.69, for serious illness, injury or assault 2.94 and for violence at home 2.16. Experiencing two or more types of trauma significantly increased the likelihood of psychosis in a dose–response relationship.
Studies by both Kraan et al and Berthelot et al in Reference Berthelot, Paccalet and Gilbert2015 again agree on an association between childhood trauma and psychosis. Kraan et al (Reference Kraan, van Dam and Velthorst2015) suggest possible mechanisms, including the formation of negative schemas and the impact of trauma on stress regulation via the hypothalamic–pituitary axis. Similarly, Berthelot et al (Reference Berthelot, Paccalet and Gilbert2015) recognise that there may be genetic or stress-related variables.
Bereavement
There is a growing body of evidence linking early stress, such as bereavement, to an increase in the later risk of psychosis. Clarke et al (Reference Clarke, Tanskanen and Hutten2013), in their study of 11 855 individuals who had lost a father or sibling before the age of 5, found a 1.6-fold increase in the odds of developing bipolar disorder and 1.3-fold increase for schizophrenia if the death was sudden (e.g. accident or suicide) rather than being a result of illness. Psychological effects on the family unit, pathological grief reactions as well as post-traumatic stress disorder (PTSD) and the biological effects of stress have all been proposed as mediating factors (Clarke Reference Clarke, Tanskanen and Hutten2013).
Violence and traumatic stress disorders
An individual with a psychotic disorder is more likely to be the victim than the perpetrator of violence. In fact, victimisation of people with psychotic disorders is 4–6 times higher than that experienced by the general community (de Vries Reference de Vries, van Busschback and van der Stouwe2019). Interestingly in this context, traumatic stress disorders (PTSD or acute stress reactions) increase the risk of schizophrenia, schizophrenia spectrum disorders and bipolar disorder. This is particularly notable in the first year following the diagnosis of traumatic stress, when there was a 15-fold increased risk. Risks are reduced but maintain significance at 5 years (Okkels Reference Okkels, Trabjerg and Arendt2017).
Bullying and negative self-schemas
A history of being bullied has been found to be common in young adults at high risk for psychosis. One study reported that 66.7% of the ultra-high-risk sample had experienced bullying, compared with 25.6% of the control group (Valmaggia Reference Valmaggia, Day and Kroll2015b). Bullying was associated with more paranoid thoughts later in life in both the control and high-risk groups. The effect of bullying on high risk of later psychosis is likely mediated by its effects on self-esteem and schema formation. Another study found that childhood memories that focused on antipathy from parents and memories of perceived threat or submission were further predictors of later paranoid ideation (Carvalho Reference Carvalho, da Motta and Pinto-Gouveia2016). Being the victim of bullying during childhood was associated with increased distress in adulthood. This helps to demonstrate the importance of the family dynamic on developing psychopathology.
Formation of negative self-schemas is linked to psychosis, as negative self-beliefs mediate the relationship between childhood trauma and paranoia. Emotional neglect was demonstrated by Appiah-Kusi et al (Reference Appiah-Kusi, Fisher and Petros2017) to be significantly associated with ultra-high-risk status, with an odds ratio of 1.33. This dropped to 1.30 when controlling for negative self-schemas; holding a negative self-schema in itself had an odds ratio of 1.19. This study found that individuals at high risk for psychosis had been exposed to higher levels of childhood trauma and had more negative core schemas compared with healthy controls. Negative self-schemas partially mediated the associations between emotional neglect and both high-risk status and paranoia.
Type and timescale of abuse
The type of abuse and timescale also carry significance. Risk of psychosis increases with greater exposure to sexual abuse; however, the risk of auditory hallucinations increases disproportionately (Sheffield Reference Sheffield, Williams and Blackford2013). Those categorised as at high risk of psychosis are more likely to have experienced their first trauma earlier in life, with one study finding that the mean age the participants experienced their first trauma was 9.8 years for the high-risk group and 16.5 years for the control group (Russo Reference Russo, Stochl and Painter2014). The high-risk individuals were also more likely to have continued to experience trauma, with multiple events throughout developmental stages, and were exposed to higher numbers of traumatic events.
Another study demonstrated that children already at familial increased risk of psychosis who were exposed to abuse or neglect had greater levels of cognitive deficits such as lower IQ (effect size ES = 0.61), visual episodic memory (ES = 0.67) and initiation (ES = 1.01) (Berthelot Reference Berthelot, Paccalet and Gilbert2015). These deficits overlap with those known to be impaired in adults with psychosis. Childhood trauma could therefore have an early mediating effect in this area.
Evidence suggests that childhood maltreatment has a structural effect on the brain. It has been associated with reduced hippocampal volume and with altered hippocampal subfield development, particularly on the left side. This area appears to be most vulnerable to the effects of abuse between the ages of 3 and 5. One particular area of interest, the subiculum, has been shown to be altered in relation to childhood maltreatment. This is important as the subiculum is linked to regulation of the hypothalamic–pituitary–adrenal (HPA) axis, dopaminergic responses associated with stress, and risk of psychosis (Teicher Reference Teicher, Anderson and Polcari2012). We will discuss these neurostructural and neurochemical components further in part 3 of this series (Romain Reference Romain, Eriksson and Onyon2019b).
Looking at the effects of adverse life events in more detail, one study reported that patients who had experienced adverse life events at the time of onset of auditory verbal hallucinations had poorer outcomes (multiple modality hallucinations and poorer general mental health), with only 9.2% of this group reporting good mental health, compared with 45.8% of those who had not experienced an adverse event at the time of hallucination onset (Bless Reference Bless, Larøi and Laloyaux2018). There may also be a cumulative risk for psychosis associated with multiple adverse events and evidence suggests a possible dose–response relationship between trauma and psychosis (van Winkel Reference van Winkel, Stefanis and Myin-Germeys2008; Sheffield Reference Sheffield, Williams and Blackford2013).
Substance misuse
Murry et al (Reference Murry, Paparelli and Morrison2013) reviewed how use of various substances has informed our knowledge of schizophrenia and psychotic symptoms. They reported that the psychotic states induced by certain drugs of misuse are related to their actions on different neurotransmitters: lysergic acid diethylamide (LSD) is thought to cause serotonergic abnormalities, amphetamines have a dopaminergic action, phencyclidine (PCP) and ketamine act on glutamate pathways, and the endocannabinoid system is activated in cannabis use. These primary pathways are considered further in part 3 of this series (Romain Reference Romain, Eriksson and Onyon2019b). Overall drug misuse has helped us to develop our current understanding of psychosis development. Murry et al (Reference Murry, Paparelli and Morrison2013) further summarised that these various drugs can mimic different aspects of schizophrenia, with stimulants and tetrahydrocannabinol (THC) being linked to paranoia, LSD more commonly associated with visual hallucinations, and ketamine and PCP with negative symptoms. Overall, they share the ability to alter experience of reality.
Cannabis
Recent statistics suggest that 6.6% of adults in England use cannabis (NHS Digital 2018). Addington et al (Reference Addington, Case and Saleem2014) conducted a review of studies investigating substance use in populations at clinical high risk for psychosis. They found that the most used substance was cannabis, with rates of 33–54%. Only one study in their review had an adequate control group with which results could be compared and this demonstrated that cannabis use in the high-risk population was significantly raised (Addington Reference Addington, Case and Saleem2014).
Multiple meta-analyses have been conducted to investigate this. An analysis of seven studies found a derived odds ratio of 2.9 when examining the relationship between cannabis use and psychosis (Semple Reference Semple, McIntosh and Lawrie2005). Marconi et al (Reference Marconi, Di Forti and Lewis2016) analysed data from ten studies (involving 66 816 individuals), finding that higher levels of cannabis use were associated with higher psychosis risk in all of the studies and giving their own odds ratio of 3.9 for schizophrenia and other psychosis-related outcomes in the heaviest cannabis users.
The precise nature of the relationship between cannabis and psychosis is less clear, with escalation in use and dose–response relationships being proposed as predictors of psychosis (Kelley Reference Kelley, Wan and Broussard2016; Kraan Reference Kraan, Velthorst and Koenders2016; Marconi Reference Marconi, Di Forti and Lewis2016). For example, a meta-analysis of seven studies conducted by Kraan et al (Reference Kraan, Velthorst and Koenders2016) suggested a dose-dependent relationship. It reported that, in an ultra-high-risk population, only cannabis use classed as misuse or dependence had a significant effect on transition to psychosis. This is contrasted by a review of ten studies in which cannabis, alcohol and nicotine were considered and which found limited evidence for an effect of increased substance use on transition from the clinical high-risk category (Addington Reference Addington, Case and Saleem2014).
A range of variables further complicates this association. For example, genetic factors influence psychosis development in cannabis users: single-nucleotide polymorphism (SNP) variation in the AKT1 gene, which is involved in dopamine signalling, increases the risk (Di Forti Reference Di Forti, Iyegbe and Sallis2012). Furthermore, accurately identifying patterns of use is difficult and the strains of the drug available on the market change over time (Gage Reference Gage, Hickman and Zammit2016). Cannabis has been linked to neurostructural changes (Abush Reference Abush, Ghose and Van Enkevort2018), behavioural problems (Ksir Reference Ksir and Hart2016) and other psychiatric disorders (Ksir Reference Ksir and Hart2016). Bizarrely, there is evidence that pre-prodromal alcohol and tobacco use have protective implications for rate of onset, and Kelley et al (Reference Kelley, Wan and Broussard2016) hypothesised that this could reflect higher social interaction and less withdrawal behaviour.
It has been noted that cannabis use has increased without a further increase documented in some psychotic syndrome diagnoses, such as schizophrenia. However, it can be argued overall that cannabis use has strong enough evidence of a link to increased psychosis risk to warrant a public health message (Gage Reference Gage, Hickman and Zammit2016).
A study by Auther et al (Reference Auther, Cadenhead and Carrión2015) demonstrated that cannabis did predict transition to psychosis in its high-risk sample, but further noted that 85% of those using cannabis were also using alcohol. At 2-year follow-up, 42.9% of those in the cannabis misuse/dependence group had developed psychosis, compared with 26.4% of non-users and 24.7% of those using cannabis without significant impairment. However, this relationship was no longer significant after adjusting for alcohol use. Similarly, alcohol was not linked to transition on its own. The authors therefore suggested a need to control for other substances in clinical studies of this topic. These would include, for example, cocaine, psychedelics, tobacco and methamphetamines, which have all been implicated in development or as confounders in other studies considered.
Comparing substance use, including alcohol, tobacco and cannabis, in a population of 731 help-seeking youths, Carney et al (Reference Carney, Yung and Amminger2017) found that individuals at risk for psychosis had significantly higher use of alcohol than individuals with no identified risk, after adjustment for age, gender and clinical variables. However, at risk status was no longer associated with alcohol use when tobacco and cannabis use were included in analysis, further reflecting the impact of polysubstance use. Other substances, such as amphetamines and opiates, were included in this study, but because of the very low use of these drugs in their population the data were not included in further analysis.
Cocaine and amphetamines
Cocaine-induced psychotic symptoms (CIPS) often occur in those taking cocaine. They most frequently include paranoid ideation and auditory and visual hallucinations. Perceptual phenomena are common during intoxication but in some individuals cocaine induces a longer-term psychotic disorder, persisting after the intoxicated period. Vergara-Moragues et al (Reference Vergara-Moragues, Gómez and González-Saiz2014) found that those with CIPS had used more cocaine and had higher dependency scores and fewer abstinence periods. They reported that 84.2% of those taking cocaine described at least one psychotic symptom in their study, contrasting this with other studies giving CIPS prevalence estimates of between 43 and 88%.
Early use and longer exposure to cocaine or methamphetamine have been found to be related to a higher severity of positive psychotic symptoms (Lichlyter Reference Lichlyter, Purdon and Tibbo2011). There are, however, some differences between these two drugs, with Alexander et al (Reference Alexander, Gicas and Willi2017) demonstrating significantly higher positive symptom scores with methamphetamine use than cocaine, although presence of psychotic symptoms in both groups was high. Concurrent cocaine and methamphetamine use did not further increase severity to a statistically significant degree. Methamphetamine has a longer elimination half-life, is more potent and has a slower clearance in the brain than cocaine. Around 50–60% of methamphetamine users experience psychotic symptoms during or after ingestion and these can include positive and negative symptoms (Alexander Reference Alexander, Gicas and Willi2017).
Methamphetamine can both precipitate and exacerbate psychotic symptoms (McKetin Reference McKetin, McLaren and Lubman2006). Methamphetamine has been shown to produce acute psychotic symptoms in trials of administration to both healthy volunteers and prior users (Glasner-Edwards Reference Glasner-Edwards and Mooney2014). However, this was not true for all participants, and time of onset following use varied, suggesting differing vulnerability to these effects. The reported vulnerability factors included symptoms of dependence, polydrug use, route of methamphetamine administration and psychiatric comorbidity. Candidate genes are also implicated in the risk of methamphetamine-induced psychosis and there is a significant overlap between higher-risk genes for methamphetamine psychosis and schizophrenia (Glasner-Edwards Reference Glasner-Edwards and Mooney2014). McKetin et al (Reference McKetin, McLaren and Lubman2006) attempted to quantify the risk. They reported that prevalence of psychotic symptoms in their sample of 309 regular methamphetamine users was 11 times higher than in the general population, and that dependent users were 3 times more likely than non-dependent users to have experienced a clinically significant psychotic symptom within the past year. Almost a quarter of the users had experienced clinically significant suspiciousness, hallucinations or unusual thought content.
LSD and ecstasy (MDMA)
Schmid et al (Reference Schmid, Enzler and Gasser2015) agreed with Murry et al (Reference Murry, Paparelli and Morrison2013) that visual hallucinations commonly occurred in their sample of 16 healthy individuals who, in a cross-over study, were given LSD. Other effects included alterations in waking consciousness, audio-visual synaesthesia, derealisation and depersonalisation. Alongside these, participants noted feelings of increased happiness, openness and trust and demonstrated physical effects such as raised heart rate and biological effects such as increased plasma cortisol, prolactin, oxytocin and adrenalin. LSD also altered sensorimotor gating, reducing pre-pulse inhibition in a manner similar to that found in established schizophrenia. The drug produces effects similar to those of other serotonergic hallucinogens such as psilocybin and N,N-dimethyltryptamine (DMT); LSD is hypothesised to act on the serotonin 5-HT2A receptor, which is upregulated in schizophrenia and is influenced by individual genetics (Schmid Reference Schmid, Enzler and Gasser2015).
Schmid et al (Reference Schmid, Enzler and Gasser2015) compared these effects with those of MDMA, which is also known to improve mood and have prosocial effects, and they noted similarities in perceptual alterations. Soar et al (Reference Soar, Turner and Parrott2001) summarised 38 case studies of patients with prominent MDMA use, finding that 29% of the individuals reported psychotic symptoms. They compared this with previous studies and reviews and concluded that individuals who have taken larger quantities of MDMA are at higher risk of psychiatric disorders, contrasting this with reports of psychotic symptoms in users that have not manifested to the extent that professional help was needed. Prospective assessment would be beneficial to further investigate this, but it raises ethical questions and difficulties as ecstasy is an illegal substance in many countries (Soar Reference Soar, Turner and Parrott2001).
Other substances
Ketamine is associated with a range of psychiatric symptoms and can trigger psychotic symptoms. Chronic ketamine users have been found to have higher levels of subthreshold psychotic symptoms. However, a recent study involving 187 chronic ketamine users found the presence of mild psychotic symptoms, but that symptoms of depression and anxiety were more dominant, with 77.5% having moderate to severe depressive symptoms and 46% moderate to severe anxiety symptoms (Fan Reference Fan, Xu and Rosenheck2016).
Another drug linked with increased risk of psychosis is PCP. This was initially marketed as a general anaesthetic, but it has been seen to induce both positive and negative symptoms of psychosis. It is thought that its effects are mediated through both dopaminergic and non-dopaminergic mechanisms (Jodo Reference Jodo2013).
Also potentially triggering psychotic symptoms are so-called ‘legal highs’. These encompass a large number of products that mimic the psychoactive effects of illicit drugs. ‘Spice’, which can contain a variety of different synthetic cannabinoids, has been particularly associated with relapse of psychotic illness and, although it was originally a legal high is now listed as a class B drug in the UK (Bajaj 2010; Every-Palmer Reference Every-Palmer2011; Zawilska Reference Zawilska2011). This may suggest that the synthetic cannabinoids carry risks of association with psychosis similar to those of non-synthetic cannabis. Other compounds that have been linked with psychosis include salvinorin A and lysergic acid amides (Zawilska Reference Zawilska2011). Mephedrone, previously a legal high but now listed as a class B drug, has also been implicated (Bajaj 2010). Although less researched, it is important to investigate legal highs to guide healthcare professionals in their response to the use of such substances (Zawilska Reference Zawilska2011).
Interestingly, case reports have been published on caffeine-induced psychosis. In one patient a psychotic episode followed an excessive caffeine intake (over 1500 mg/day for 2 days). The patient presented with paranoid delusions and the incident culminated with him accidentally shooting himself in the chest. Together with genetic vulnerability factors, one proposed mechanism of action of caffeine is through adenosine receptor antagonism, increasing dopamine neurotransmission (Goiney Reference Goiney, Gillaspie and Alvarez Villalba2012). Caffeine also affects noradrenalin release, which may imply a role in inducing anxiety symptoms. There is no clear consensus on the role of caffeine in psychotic manifestations and necessary doses needed to provoke symptoms have not been established (Wang Reference Wang, Woo and Bahk2015). This research is, however, in its early days.
Sleep
Sleep disturbance is highly prevalent among people with schizophrenia, including longer sleep-onset latency, greater disruption in sleep continuity and day/night reversal, and it is associated with a higher symptom burden. Evidence further suggests that sleep disturbance is a common prodromal symptom, but there is no firm evidence that any particular individual element of sleep disturbance predicts transition to psychosis. It has been shown that those at clinical high risk of psychosis demonstrate higher levels of sleep disturbance and this has been suggested as a possible future target for treatment (Poe Reference Poe, Brucato and Bruno2017).
A study using actigraphy (a method of collecting sleep data in a more natural environment than polysomnogram) to investigate sleep patterns in adolescents at ultra-high risk of psychosis found that certain sleep elements – in this case, greater numbers and duration of awakenings, increased movement and decreased sleep efficiency – correlated with positive psychotic symptoms (Lunsford-Avery Reference Lunsford-Avery, LeBourgeois and Gupta2015). The authors noted that sleep difficulty might be linked to psychosis through a variety of mechanisms, including increased stress, worsened cognition and neurodevelopmental changes.
Sleep dysfunction has also been linked to negative symptoms. A study using structural MRI found that ultra-high-risk participants had decreased grey volume of the thalamus bilaterally, associated with greater sleep dysfunction (Lunsford-Avery Reference Lunsford-Avery, Orr and Gupta2013). Sleep is thought to be a key factor in synaptic plasticity, which plays a role in sleep-dependent memory consolidation. Functional connectivity in the hippocampus is greater during non-rapid eye movement sleep. In schizophrenia, patients demonstrated reduced density, number and coherence of sleep spindles on an electroencephalogram (EEG), features linked to plasticity and cognitive impairments (Keshavan Reference Keshavan, Mehta and Padmanabhan2015).
In a study attempting to identify whether insomnia could be a causal factor for psychotic symptoms, a group of healthy volunteers had one 3-day period of normal sleep (average 6 h 58 min) and one of reduced sleep (average 5 h 15 min) and were measured following each for psychotic symptoms. After the reduced-sleep period, participants showed higher levels of paranoia (ES = 0.383), hallucinations (ES = 0.869) and cognitive disorganisation (ES = 0.643), suggesting that insomnia might have a causal role in psychosis. They further reported increased distress in relation to psychotic experiences (ES = 0.521) (Reeve Reference Reeve, Emsley and Sheaves2017).
A systematic review has corroborated co-occurrence of sleep disturbance and psychotic experiences, suggesting that sleep dysfunction has a role in predicting psychotic experiences and that improving sleep in those with psychosis may lessen symptoms (Reeve Reference Reeve, Sheaves and Freeman2015). In their analysis the authors reported key figures such as an odds ratio of 1.78–2.54 for insomnia with concomitant paranoia and an odds ratio of 1.49–1.84 for insomnia predicting new paranoid thinking. To address sleep dysfunction in an attempt to improve psychosis, specific therapies such as cognitive–behavioural therapy for insomnia (CBT-insomnia) could be a useful adjunct to treatment (Lunsford-Avery Reference Lunsford-Avery, LeBourgeois and Gupta2015; Reeve Reference Reeve, Sheaves and Freeman2015).
Conclusions
This article, the second in a three-part series, has discussed the most up-to-date evidence for risk factors for psychosis symptom development that affect individuals primarily during later childhood, adolescence and adulthood. Potential risk factors are summarised in Box 2, but it should be noted that the evidence base for some of these is sparse. The next and final article in the series (Romain Reference Romain, Eriksson and Onyon2019b) focuses on: the final common pathways leading to psychosis; neurochemistry, neurostructure and inflammation; and possible primary, secondary and tertiary preventive strategies.
Box 2 Potential psychosis risk factors in adolescence and adulthood
Living environment
• Urban living
• Low socioeconomic status
• Frequent environment change
• Migration
• Being a refugee
Communicative environment
• Parental schizophrenia
• Poor parent–child relationships
• Social defeat (prolonged exposure to social exclusion or adversity)
• Social cognition and emotion
• Impaired theory of mind
• Poor affect recognition
• Thought disorder, reduced ideational richness
• Impaired motivation and isolation
Psychopathology
• Depression and anxiety disorders
• Behavioural problems
• Altered personality profiles (e.g. higher harm avoidance; lower reward dependence, self-directedness and cooperativeness)
• Impaired neurocognitive function
Stigma
• Feelings of anxiety related to at-risk label and symptoms of psychosis
Trauma and abuse
• Sexual, physical or emotional abuse
• Early stress such as bereavement
• Traumatic stress disorders (PTSD or acute stress reactions)
• Being the victim of bullying
• Formation of negative self-schemas
Substance (drug) misuse
• Cannabis, cocaine and amphetamines, LSD and ecstasy (MDMA) and other substances
Sleep disturbance
MCQs
Select the single best option for each question stem
1 The active ingredient in cannabis is:
a TBA
b THC
c CTA
d CGG
e cannabin.
2 Which of the following is not a risk factor for psychosis?
a migration
b urban environment
c high levels of pollution
d high socioeconomic status
e moving house often during adolescence.
3 Parental communication deviance refers to:
a parents’ use of vague, fragmented or contradictory language
b parents’ use of derogatory terms about their children
c a communication difficulty arising from an intergenerational gap
d particularly anachronistic parental language
e particularly contemporary parental language.
4 In a youth population, sexual abuse has been estimated to increase the risk of diagnosis of a later psychotic disorder by:
a 10 times
b 100 times
c 2 times
d 5 times
e 15 times.
5 The primary pathway amphetamines are thought to act on in causing psychotic symptoms is:
a serotonin
b cannabinoid
c dopamine
d glutamate
e adrenalin.
MCQ answers
1 a 2 d 3 a 4 a 5 c





eLetters
No eLetters have been published for this article.