Abstract
Background
Protein C deficiency is a rare genetic disorder with varying severity of symptoms and disease. The disorder may vary in presentation from a complete symptomless state to a less severe form like venous thromboembolism. The most severe form of disease is a rare condition called neonatal purpura fulminans (NPF) which is characterized with sudden progressive dermal hemorrhage and necrosis due to vascular thrombosis and disseminated intravascular coagulation. In contrast, congenital atrial and ventricular septal defects are the commonest congenital heart diseases found in pediatric population. An infant presenting with systemic vascular thromboembolism secondary to protein C deficiency along with the cardiac septal defects posted for surgery will be a very challenging task to manage in perioperative period. Also, physiological mechanisms during perioperative period and surgery will promote thromboembolism leading to worsening of the situation further. So, perioperative management of such patient pose a great challenge to the anaesthesiologist. Due to rarity of the condition, there is very limited literature available.
Case presentation
We report the perioperative management of a 2-month-old child suffering with neonatal purpura fulminans with atrial and ventricular septal cardiac defect, scheduled for bilateral foot amputation. The patient was a diagnosed with complete occlusion of abdominal aorta leading to foot gangrene. After initiation of anticoagulant therapy, symptoms were relieved and patient was posted for amputation of gangrenous feet.
Conclusions
There could be an increased risk of thromboembolism and bleeding due to protein C abnormality along with the chances of shunt reversal, paradoxical embolism, and other cardiac morbidities secondary to septal defects. Wise selection of anaesthetic agents like limiting the use of nitrous oxide, ketamine as much as possible to be considered. Conditions like tachycardia, hypotension, and hypothermia should also be prevented perioperatively as these could increase the chances of thrombosis.
Similar content being viewed by others
Background
Protein C deficiency is very rare form of bleeding disorder with varying incidence of mild, moderate, and severe form of disease. It may range from a complete asymptomatic state to venous thromboembolism leading to disseminated intravascular coagulation in severe state. Whereas, the most severe form of the disease presents as a rare, lethal condition called as neonatal purpura fulminans (NPF). On the other hand, atrial and ventricular septal defects are the most common congenital cardiac defects present worldwide. Presence of both the diseases in an infant simultaneously who is scheduled for surgery poses a great challenge to the anaesthesiologist. Due to rarity of the condition, there is very limited literature available and this case report is first of its kind. Here, we report the perioperative management of an infant having neonatal purpura fulminans due to congenital protein C deficiency with atrial and ventricular septal cardiac defect.
Case presentation
A 2-month-old male child was scheduled for bilateral foot amputation following progressive peripheral gangrene secondary to protein C deficiency. Pre-anaesthetic evaluation revealed that the healthy infant was born at full term with normal vaginal delivery. After 15 days of birth, mother noticed bluish discolouration of the skin over the right toe of the child. It was very rapidly progressive with extension up to complete dorsal surface and sole of bilateral feet along with formation of blisters and edema over the affected area. Patient approached to the pediatric casualty after 5 days of onset of progressive discolouration of bilateral lower limb with high grade fever and decreased oral intake. On examination, the baby was lethargic and cyanosed with a pulse rate 160 beats per minute, respiratory rate 77 breaths per minutes and body temperature 103 °F with absence of femoral pulse in both the limbs. On systemic examination, no other significant findings were present.
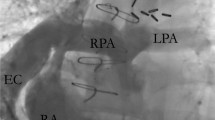
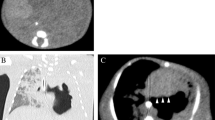
The pediatric team considered numerous differential diagnoses including aortic thrombosis or embolism, septic thrombosis or embolism, distal aortic or femoral arterial stenosis, umbilical artery catheterization and congenital hypercoagulable states. The child was investigated on the lines of forementioned causes of presentation. Sepsis workup including blood culture and cerebrospinal fluid examination was done at the time of admission following which suitable antibiotics along with the supportive treatment were started immediately. Also, color doppler scan of bilateral lower limbs was performed which was suggestive of acute thrombosis with total occlusion of abdominal aorta commencing just beyond the level of right renal artery and extending in the bilateral common iliac arteries leading to partial occlusion of the arteries with no colour flow in the arteries of both lower limbs. Echocardiography was also performed to rule out any emboli which revealed atrial septal defect of ostium secundum (size 2.6 mm) and ventricular septal defect (size 1.4 mm) with left to right shunt. Coagulation profile was also done to rule out thrombophilia and hypercoagulable states which suggested severely decreased values of protein C and normal values of protein S, Anti thrombin, and Factor V Leiden.
After considering protein C values, Injection (inj.) Enoxaparin 2.5 mg subcutaneous was started twice a day. After 10 days, arterial flow was improved as compared to the previous scan. There was no further extension of the lesions except for the progression of existing lesion to a state of wet gangrene associated with discharge. After 20 days, there was appearance of demarcation line and the patient was scheduled for amputation of the feet along with stump formation under general anesthesia as values of protein C improved from 5.3 to 46.8% (normal level 80–130%) along with other normal coagulation parameters. Inj. Enoxaparin was stopped 12 h prior to surgery and blood products were cross matched.
After taking informed written consent about the disorder and associated complications like excessive bleeding and perioperative systemic thromboembolism etc. Patient was taken to the operation theater where a temperature of 24 °C was maintained. Baseline readings of vital parameters like SpO2 (oxygen saturation), electrocardiogram (ECG), non-invasive blood pressure (NIBP) and temperature were recorded. General anaesthesia was induced with inhalation of sevoflurane in 100% oxygen in titrated doses with special focus on avoiding vigorous ventilatory efforts. Simultaneously, peripheral venous cannula is secured and calculated volume of intravenous fluid is administered via burette set after ensuring absence of any air bubbles. Inj. fentanyl (5 mcg.kg−1) was given to ease the tracheal intubation. Airway was successfully secured with an uncuffed endotracheal tube (3.5 mm internal diameter) after gentle laryngoscopy. Anesthesia was maintained with inhalational mixture of oxygen with sevoflurane while refraining the use of nitrous oxide. Intraoperatively, inj. fentanyl (2 µg.kg−1) and inj. atracurium (0.5 mg.kg−1 as loading dose, 0.1 mg.kg−1 as maintenance dose) intravenous were repeated regularly on demand. SpO2, ECG, NIBP, end tidal CO2, and temperature (rectal) were monitored continuously. The patient was ventilated on pressure control mode with aim of delivering the tidal volume of around 6 ml.kg−1 and a respiratory rate 40 per minute. Normothermia was maintained with the help of forced air warmer. Ringer acetate is used as maintenance fluid (10 ml.h−1 including drug solutions) along with blood loss replacement with the same (around 25 ml) intra-operatively (calculated according to Holliday-Segar method). The entire procedure involving debridement of the necrosed tissue and stump formation lasted for about 45 min and was uneventful. After completion of the surgery, reversal of neuromuscular blockade was achieved with inj. neostigmine (0.05 mg.kg−1) and inj. atropine (0.02 mg.kg−1). After achieving adequate signs of reversal trachea was successfully extubated. The patient was comfortable, warm and awake. Antibiotic therapy was continued in the postoperative period and inj. Enoxaparin was started again after 6 h of surgery. Coagulation profile was repeated regularly in postoperative period which was suggestive of higher values of protein C as compare to previous values. There was a serial improvement in levels of protein C before and after the surgery as shown in the blood tests. This improvement might be due to the treatment of sepsis with the final exclusion of septic foci from the body after amputation. The child remained clinically stable and was discharged on 5th postoperative day. Patient was advised to follow-up in the pediatric, hematology, and plastic surgery departments.
Discussion
Protein C is a vitamin K-dependent glycoprotein which is normally present in the plasma in its inactive form (Zymogen) and functions as a co-factor in regulating the process of thrombogenesis. It primarily downregulates the process of thrombin generation which is initiated by activated factor V and VII, after cleavage at the specific sites of these factors (Patil and Sabu 2017). Its activity is further dependent on many other factors present in the plasma like protein S, high-density lipoprotein, and anionic phospholipids.
Protein C deficiency is a rare form of disorder with the varying incidence of severity. The cause of disease could be either genetic or acquired with former being more common. The disorder is expressed as an autosomal-dominant trait, where homozygotes generally fail to survive even during the period of infancy due to life threatening condition like massive systemic thrombosis. Whereas, patients with homozygous and compound heterozygous protein C deficiency might present with fatal neonatal purpura fulminans (NPF). Individuals with heterozygotic trait generally present with the less severe episodes of thrombosis with the age of presentation during their mid-twenties. Acquired form of disease is very rare and occurs due to severe compromise of hepatic function (Sahoo and Jagannathan 2015).
Also, activated protein C is known to play a major part in regulating the cell inflammation (Yen 2015). It binds to endothelial protein C receptor and endothelial cell protease-activated receptor-1. This bonding supresses proinflammatory and proapoptotic mediator activity along with promotion of anti-inflammatory and antiapoptotic pathways (Tiwari and Tomer 2012). As concluded from the above discussion, patients with protein C deficiency have impaired regulation of antithrombotic process present in the body under normal physiological conditions. Therefore, most common complication and presentation associated with protein C deficiency is vascular thromboembolism which in extreme cases might lead to purpura fulminans. Treatment of protein C deficiency depends on the severity of symptoms. Acute cases are treated with protein C supplementation by administration of human plasma derived viral-inactivated protein C concentrate combined with the anticoagulants like low molecular weight heparins, warfarin. Whereas, mild to moderate cases can be managed with anticoagulants alone (Tiwari and Tomar 2012). However, this critical case is manged with low molecular weight heparin alone due to non-availability of nearby government aided free/low-cost centers and the higher cost associated with the processed protein C concentrate in the market.
Special care must be taken in these cases while considering these patients for anaesthesia. The choice of anaesthetic technique must be atraumatic to avoid disease specific complications like bleeding and thrombosis. Cuffed endotracheal device may increase the risk of tracheal compression with subsequential submucosal thrombosis and necrosis. Also, tissue compression and dehydration aggravate the risk of thrombosis. All the pressure points must be padded with soft cotton to prevent tissue compression. The patient must be adequately hydrated with crystalloids and blood products to overcome dehydration as it leads to increased risk of thromboembolic phenomenon. Also, surgical stress and trauma will further aggravate the thrombogenic process of the body during perioperative period. Prevention of hypothermia is also very important in these cases, as hypothermia may trigger thrombosis (Miller et al. 2000).
Our case also had atrioventricular septal defects which could further fire up the situation. Both the atrial and ventricular septal defects together constitute majority of congenital heart diseases among children (Yen 2015). Major direction of shunting in both the defects is from left to right heart. Anaesthetic management of an ASD and VSD focuses on reducing the magnitude of the shunt and also preventing the establishment of reversal of shunt in severe cases (right to left shunt). Systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) are 2 main parameters to decide direction and magnitude of the shunt and are commonly affected during sedation and anaesthesia (Yamamoto and Schindler 2016). So, major attention should be given to prevent conditions like vigorous ventilatory efforts, laryngospasm and bronchospasm to limit the increase in PVR because acute increase in PVR can lead to reversal of shunt direction. Similarly, a state with very low SVR as compare to PVR should also be avoided which happens during deep anaesthesia with hypovolemia and hypotension. In our case, excessive risk of thromboembolism is also posing the patient on increased risk paradoxical embolism due to septal defects.
Conclusions
It is concluded that cases with rare diseases like protein C deficiency with congenital atrial and ventricular septal defect pose a great challenge to anaesthesiologist. There could be an increased risk of thromboembolism and bleeding due to protein C abnormality along with the chances of shunt reversal, paradoxical embolism and other cardiac morbidities. So, goal of anaesthetic management should ideally be maintenance of SVR and PVR. Also, introduction of air bubbles to the circulation through intravenous lines should be avoided. Wise selection of anaesthetic agents like limiting the use of nitrous oxide, ketamine as much as possible to be considered. Conditions like tachycardia, hypotension, and hypothermia should also be prevented perioperatively as these could increase the chances of thrombosis. Another finding that needs mention is that a severe case of protein C deficiency can be managed with anticoagulants alone in a limited resources facility.
Availability of data and materials
Not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- NPF:
-
Neonatal Purpura Fulminans
- inj.:
-
Injection
- mg.kg−1 :
-
Milligram per kilogram
- ml:
-
Milliliter
- SVR:
-
Systemic vascular resistance
- PVR:
-
Pulmonary vascular resistance
References
Miller RD (2000) Anesthesia, vol 1, 5th edn. Churchill Livingstone Publishing; p, New York, p 1150
Patil AD, Sabu J, D’Souza O (2017) Anesthesia management of the parturient with protein S and C deficiency for caesarean section. Journal of Anaesthesia and Critical Care Case Reports 3(3):14–15
Sahoo RK, Jagannathan B, Palanichamy G, Nair AS (2015) Management of perioperative anticoagulation in a patient with protein ‘C’ deficiency and multiple comorbidities for spinal surgery. Anaesth Pain & Intensive Care 19(4):495–498
Tiwari AK, Tomar GS, Tayal S, Chadha M, Kapoor M (2012) Anaesthetic significance and management of a child with neonatal purpura fulminans. Indian J Anaesth 56:283–286
Yamamoto T, Schindler E (2016) Anaesthesia for non-cardiac surgery in children with congenital heart disease. Anaesthesiology Intensive Therapy 48(5):305–313
Yen P (2015) ASD and VSD Flow Dynamics and Anesthetic Management. Anesthesia Progress 62:125–130
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
MG contributed to the care of the patient, acquisition of data, and drafting of the manuscript. AT contributed to critical revision and final approval of the version of manuscript to be published. AL contributed to the care of the patient and acquisition of data published in the manuscript. MG contributed to the drafting, revision, and final approval of the version of manuscript to be published. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, because our manuscript is a case.
Consent for publication
Written informed consent from the parents of the patient was obtained for the publication of the case report including case details and images in the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, M., Tiwari, A. & Lather, A. Anaesthetic management of an infant with severe protein C deficiency and septal cardiac defects—a case report. Ain-Shams J Anesthesiol 15, 43 (2023). https://doi.org/10.1186/s42077-023-00342-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-023-00342-6