Abstract
Background
This study aimed to compare the duration of postoperative analgesia using intraperitoneal bupivacaine plus neostigmine or bupivacaine alone. In this randomized controlled trial, we included 56 adult patients scheduled for elective laparoscopic cholecystectomy operation. Patients were randomly allocated into two groups bupivacaine group (B group) who received 50 ml bupivacaine 0.25% alone and bupivacaine-neostigmine group (BN group) who received 500 μg neostigmine added to 50 ml bupivacaine 0.25%. The study drug was instilled intraperitoneal according to group allocation before the start of the surgery. Primary outcome was the duration of analgesia. Other outcome included total dose of postoperative analgesic consumption and postoperative nausea and vomiting.
Results
Patients in BN group showed longer duration of analgesia after surgery and longer time for 1st analgesic dose than the patients in B group. Total dose of postoperative pethidine and the incidence of postoperative nausea and vomiting were lower in number in the BN group than in the B group.
Conclusions
Intraperitoneal instillation of neostigmine as an adjunct to bupivacaine in elective laparoscopic cholecystectomy increased the duration of postoperative analgesia. Also, it might reduce postoperative pain and analgesic requirements as compared to bupivacaine 0.25% alone.
Trial registration
Name of the registry: Clinical trial
Clinical Trial registration number: NCT04244097
Date of registration: 28 January 2020
URL of trial registry record: https://clinicaltrials.gov/ct2/show/NCT04244097
Similar content being viewed by others
Background
Laparoscopic cholecystectomy (LC) is the standard technique for gall bladder surgeries nowadays (Toleska et al., 2018).
Although pain after LC is less severe than after open cholecystectomy (OC), some patients are suffering from discomfort and pain during the first 24 to 72 postoperative hours (Alam et al., 2009).
Opioids were prescribed to control acute pain postoperatively, but it was found that opioids increase the incidence of postoperative vomiting (Hsieh et al., 2021). So it was recommended to use opioids only when other analgesic methods fail (Bisgaard & Warltier, 2006).
Transversus abdominis muscle block a good method to control postoperative somatic pain; however, this technique lacks visceral pain control (Petersen et al., 2012).
Several studies reported the analgesic effect of local anesthetics when injected intraperitoneally (Toleska et al., 2018; Bisgaard & Warltier, 2006; Arora et al., 2019) being, simple, and effective technique with minimal side effects.
Local anesthetics inhibit nociception by their effect on the nerve membrane-associated proteins and inhibit the release and action of prostaglandins and other agents that sensitize or stimulate the nociceptors and contribute to inflammation.
Neostigmine is a choline esterase inhibitor; a number of studies have investigated the intrathecal, epidural, caudal, and intra-articular routes of administration of this agent, as well as the addition of neostigmine to local anesthetics used for brachial plexus block and intravenous regional anesthesia. Overall, it appeared to improve postoperative analgesia in most studies without increasing the incidence of adverse events (Booth et al., 2017; Abu et al., 2017; Habib & Gan, 2006).
This study was designed to assess the clinical efficacy of neostigmine as an adjunct to intraperitoneal bupivacaine for the relief of postoperative pain after laparoscopic cholecystectomy (LC) versus the use of bupivacaine alone.
Methods
This randomized controlled trial was conducted in Cairo University Hospital between April 2020 and August 2020, after obtaining approval from the institutional (Kasr Alainy, Faculty of Medicine) research ethics committee (MS-273-2019) and clinical trial registration (NCT04244097). Patients were randomly allocated to two groups based on computer-generated random number tables in opaque-sealed envelopes prepared by an anesthesiologist not part of the study.
All patients included in the study were aged 18 to 60 years, American Society of Anesthesiologists (ASA) physical status I-II, scheduled for elective laparoscopic cholecystectomy and with body mass index (BMI) less than 35.
While any patient refused to participate, ASA III-IV, patient allergic to local anesthesia or neostigmine or presented with acute cholecystitis, were excluded from the study.
-
Bupivacaine group (B group) received a 50 ml solution of bupivacaine 0.25% intraperitoneal instilled solution.
-
Bupivacaine neostigmine group (BN group) received 500μg neostigmine (Yang et al., 1998) mixed with bupivacaine 0.25% with a total volume of 50ml intraperitoneal instilled solution (with a maximum dose of 2mg/kg bupivacaine in both groups). The envelopes were opened by the staff nurse, and peritoneal solution was prepared according to group allocation by another anesthesia assistant nurse who is not involved in the study.
In the pre-anesthetic room, baseline hemodynamics were recorded, heart rate (HR), and mean arterial blood pressure (MAP). An intravenous access was secured; all patients received premedication just before surgery with midazolam 0.05 mg/kg, ranitidine 50 mg, and ondansetron 0.15 mg/kg over 15 min intravenously. The patient then transferred to the operating room where full monitors were applied (ECG, pulse oximeter, noninvasive blood pressure monitor, and capnography were added after endotracheal tube insertion).
Preoxygenation with 100% oxygen for 3 min and induction of general anesthesia intravenously were done with propofol 2 mg/kg, fentanyl 2 μg/kg, and atracurium 0.5 mg/kg followed by orotracheal intubation after 3 min of positive pressure ventilation. Before the start of surgery, after insertion of the trocars and inflating the peritoneum, before any surgical manipulation, the surgeon infused the study drug (according to the group allocation) intraperitoneally to the subdiaphragmatic space and gall bladder area guided by the camera, while the patients were kept in Trendelenburg position for 5–10 min. Thereafter, all patients were positioned in the anti-Trendelenburg position to start the surgery, and the laparoscopic procedure was carried out in a standard fashion. Maintenance of anesthesia was done using isoflurane 1.2% in oxygen/air (60/40%) mixture. Increments of 0.1 mg/kg atracurium were administered repetitively every 20 min to achieve muscle relaxation.
Baseline hemodynamics were recorded, HR and MAP 1 min before induction of anesthesia, and then recorded on a chart every 15 min. When MAP or HR raised of > 20% of baseline, we administered 0.5 μg/kg intravenous bolus of fentanyl.
During laparoscopy, intra-abdominal pressure was maintained between 10 mmHg and 15 mmHg. Minute ventilation volume was adjusted to keep end-tidal PCO2 between 35 and 40 mmHg. At the end of the surgery, isoflurane discontinued; FIO2 was increased to 80%. The residual neuromuscular blockade was reversed with a mixture of neostigmine 0.05 mg/kg and atropine 0.01 mg/kg, and then extubation was done. The time of arrival to the postanesthesia care unit (PACU) is defined as 0 h postoperatively. All patients stayed in PACU after surgery for 2 h before transferral to the ward. A fixed dose of intravenous paracetamol 1 g was given every 6 h to all patients in both groups starting from 0 h in PACU. In case of VAS, pain score ≥ 4 or patient request for analgesia, a 25 mg pethidine (increased to maximum 100 mg/dose when required) was given intravenously every 4 h to keep the VAS score less than 4 with maximum dose of 600 mg/day. Patient was observed for nausea and vomiting, and if there was severe nausea or vomiting, ondansetron 4 mg was given intravenously (with a maximum dose of 16 mg/day) [postoperative nausea and vomiting were rated on a 4-point scale (0 = no PONV, 1 = mild nausea, 2 = severe nausea, 3 = vomiting) and were assessed and recorded].
The primary outcome was the time of first analgesic requirement (defined as time from injecting local anesthetic intraperitoneal until requesting the first postoperative analgesia).
Secondary outcomes included number of patients requiring intraoperative fentanyl and postoperative pethidine, total dose of postoperative pethidine consumption (mg/24 h), hemodynamics (HR and MAP measured at baseline, every 15 min intraoperatively, and at 1, 2, 6, 12, and 24 h postoperatively), incidence of bradycardia (HR < 60 bpm), hypotension (MAP < 80% of baseline), and hypertension (MAP > 120% of baseline), postoperative shoulder and abdominal pain assessed by VAS (at 1, 2, 6, 12, and 24 h postoperatively), and postoperative nausea and vomiting.
Statistical analysis
Sample size was calculated using G*Power 3.1.9.2, regarding our primary outcome; time of first analgesic requirements (in hours) after extubation based on previous study (R. H. Mostafa et al. 2018) was (7.73 ± 1.87 and 20.26 ± 0.835 h in B group [bupivacaine] and BK group [bupivacaine + ketamine], respectively) (Mostafa & Mekki, 2018). Power analysis was performed using the t-test (Student’s t-test) for independent samples. Taking power 0.8 and alpha error 0.05 and predicting a mean difference of 20% between both groups, a minimum sample size of 25 patients is calculated for each group. A total of patients in each group 28 will be included to compensate for possible dropouts.
Data was coded and entered using the statistical package SPSS version 26 (SPSS Inc., Chicago, IL, USA). Data were summarized using mean and standard deviation or median and quartiles for quantitative variables as appropriate and frequencies (percentages) for categorical variables. Continuous data were checked for normality using the Shapiro-Wilk test and presented as mean (standard deviation) or median (quartiles). Normally distributed data were analyzed using the unpaired Student t-test, while skewed data were expressed as medians (quartiles) and were analyzed using the Mann Whitney U-test. For repeated measures, the repeated measure analysis of variance (ANOVA) was used to evaluate drug (between-groups factor) and time (repeated measures). Bonferroni test was used to adjust for multiple comparisons P-value of 0.05, or less was considered significant.
Results
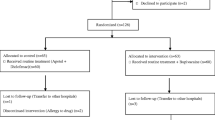
Sixty patients were screened for eligibility. Four patients were excluded for not fulfilling our inclusion criteria. Fifty-six patients were included and were randomized into either B group (n = 28) or BN group (n = 28). All patients received the assigned treatment and were available for the final analysis (Fig. 1).
Demographic data and baseline hemodynamic characteristics were comparable between both groups (Table 1).
Time to first analgesic requirement was longer in BN group than in B group (median [quartiles]: 12 (Arora et al., 2019; Boogmans et al., 2014) h and 3 (Alam et al., 2009; Arora et al., 2019) h, respectively, P-value: 0.001). Furthermore, the total dose of postoperative pethidine was lower in BN group than in B group. However, the number of patients who needed supplemental fentanyl analgesia intraoperative and pethidine postoperatively was comparable between both groups (Table 2).
Furthermore, postoperative abdominal VAS at rest and during movement as well as shoulder VAS were generally comparable between both groups (Table 3).
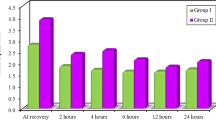
The heart rate decreased intra- and postoperatively in relation to the baseline reading in each group and was lower in the BN group than in the B group postoperatively (Fig. 2).
The MAP was maintained intraoperatively in both groups. Postoperatively, the MAP in the BN group decreased in comparison with the baseline reading and was lower than that of the B group (Fig. 3).
Seven (25%) patients in BN group and 5 (18%) patients in B group developed hypotension, P-value: 0.515. On the other hand, 5 (18%) patients in BN group and 7 (25%) patients in B group developed hypertension, P-value: 0.515 (Table 2).
The incidence of postoperative nausea and vomiting was likely lower in the BN group (7 patients [25%]) than in B group (14 patients [50%]), P-value = 0.053 (Table 2).
Discussion
In this study, we compared bupivacaine versus bupivacaine-neostigmine admixture when injected intraperitoneal in elective laparoscopic cholecystectomy surgery. It was found that postoperative analgesia and time to first analgesic requirement were longer in BN group than in B group (median [quartiles]: 12 (Arora et al., 2019; Boogmans et al., 2014) h and 3 (Alam et al., 2009; Arora et al., 2019) h, respectively, P-value: 0.001).
Furthermore, the total dose of postoperative pethidine was lower in BN group than in B group (25 [0, 25], 50 [25, 50], respectively, with P-value 0.002). However, the difference between both groups regarding the number of patients who needed supplemental analgesia intra- and postoperatively was not statistically significant.
Regarding postoperative abdominal VAS at rest and during movement as well as shoulder, VAS were comparable in both groups. Incidence of hypertension and postoperative nausea and vomiting was lower in the BN group although was not significant statistically.
Neostigmine as cholinesterase inhibitor was used in many studies for its analgesic effect on muscarinic peripheral receptors. The peripheral administration of neostigmine has minimal or no centrally mediated side effects in comparison with its neuraxial use (Yang et al., 1998).
Up to our knowledge at the time of the study, no previous studies used neostigmine alone or as adjuvant with local anesthetics for intraperitoneal installation, yet it was used in many other sites (Booth et al., 2017; Abu et al., 2017; Habib & Gan, 2006; Yang et al., 1998).
In consistence with our study, Yang et al. included 60 patients having arthroscopic meniscus repair in their study. Patients were randomized into 6 groups: 10 patients for each to receive after the operation 125, 250, and 500 μg intra-articular neostigmine, 2 mg intra-articular morphine or as control groups intra-articular saline, or 500 μg neostigmine given subcutaneously (SC). They showed that analgesia lasted longer after 500 μg intra-articular neostigmine (350 ± 126 min) compared with intra-articular morphine (196 ± 138 min, P < 0.05) or with intra-articular saline (51 ± 11 min, P < 0.05). They also found that the total amount of postoperative rescue morphine was significantly higher in the control groups than for patients given intra-articular 500 μg neostigmine. In line with our study, they found that intra-articular (500 μg) neostigmine resulted in significant VAS reduction 1 h after injection compared with patients given intra-articular saline and with those given intra-articular morphine (P < 0.05). Also, there were no observed side effects (nausea and vomiting) among all study groups (Yang et al., 1998).
In consistence with ours, Thomas Boogmans et al. included 100 healthy, term (37 weeks) parturients who had requested regional analgesia during labor (combined spinal-epidural block). The epidural study solution contained 10 ml physiological (“normal saline”; 0.9% saline) placebo group (group P) or a mixture of clonidine 75 mg and neostigmine 500 μg dissolved in 10 ml saline (group NC). Patients were assigned randomly to one of the two study groups. Pain was assessed at the moment the patient reported breakthrough pain. A significant difference between both groups in breakthrough pain was noted: only 6% of patients in group NC had breakthrough pain compared with 36% in the group P (P < 0.001). Also, patient satisfaction in the 1st hour after labor was significantly better in group NC than in group P (P < 0.05) (Boogmans et al., 2014).
In line with our study in Bone et al., included 34 patients undergoing elective surgery on the upper extremity under axillary brachial plexus block anesthesia were equally divided into two groups; group M received an axillary brachial plexus block with mepivacaine 500 mg (50 ml) and isotonic saline (1 ml), and group NM received an axillary brachial plexus block with mepivacaine 500 mg (50 ml) and 500 μg neostigmine (1 ml).
The onset and duration of sensory and motor block were comparable between both groups. Patients in NM group recorded lower pain scores ((VAS): 14.7 ± 9.9 vs 32.4 ± 23.5; P < 0.05) 24 h after surgery; also, lower patients in the NM group needed analgesics supplementation during the first 24 h after surgery. No adverse events (nausea or vomiting) were recorded for both groups (Bone et al., 1999).
In the study of Mostafa RH et al., 40 patients of both sex planned for elective LC. After inflating the peritoneum, the surgeon sprayed 50 ml of a blinded solution intraperitoneally. Patients were randomly allocated into the following: group B received a 50 ml of intraperitoneal bupivacaine 0.25%, and group BK received 0.5 mg/kg ketamine mixed with bupivacaine 0.25%. Their results showed that ketamine bupivacaine admixture caused a dramatic decline in shoulder pain VAS scores especially at the 24th h; 15 patients in the BK group had either VAS score zero or 1 when compared to B group whom their lowest score at the 24th h was 4. Also, there was marked decrease in postoperative analgesic consumption in BK group (Mostafa & Mekki, 2018).
In the study done by Atia and Abdel-Rahman, 80 patients who were scheduled for elective hand and forearm surgery under intravenous regional anesthesia (IVRA) were divided into two groups, The control group (group C) received 3 mg/kg 0.5% lidocaine plus 1 ml normal saline, while the neostigmine group (group N) received 3 mg/kg 0.5% lidocaine plus 1 mg neostigmine. They found no significant difference between the two groups as regards the time to first analgesic request, total postoperative ketorolac consumption, and the number of patients who requested pethidine. It was concluded that addition of 1 mg neostigmine to 0.5% lidocaine in IVRA has no analgesic or anesthetic effect (Atia & Abdel-Rahman, 2016)
In the review article done by Cossu A. P. et al., comparison of neuraxial administration of neostigmine or neostigmine with local anesthetics and/or other adjuvants versus placebo or placebo with local anesthetics and/or other adjuvants studies was analyzed. The addition of neostigmine in reduces the dose of local anesthetic during labor analgesia and postoperative analgesia following cesarean section: mean reduction of local anesthetic (ropivacaine or bupivacaine) vs. control (P = 0.002). Although the risk of nausea and vomiting was increased with intrathecal administration (neostigmine 72/110 vs. control 22/125, P < 0.001) but not with epidural administration (neostigmine 31/309 vs. control 13/167, P = 0.94) (Cossu et al., 2015).
Conclusions
Intraperitoneal instillation of neostigmine as an adjunct to bupivacaine in elective laparoscopic cholecystectomy increased the duration of postoperative analgesia. Also, it might reduce postoperative pain and analgesic requirements as compared to bupivacaine 0.25% alone.
Limitations
Different doses of neostigmine as adjuvant to bupivacaine can be compared in further studies.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- B group:
-
Bupivacaine group
- BN group:
-
Bupivacaine-neostigmine group
- LC:
-
Laparoscopic cholecystectomy
- OC:
-
Open cholecystectomy
- ASA:
-
American Society of Anesthesiologist
- BMI:
-
Body mass index
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- PACU:
-
Postanesthesia care unit
- VAS:
-
Visual analogue scale
- PONV:
-
Postoperative nausea and vomiting
- IVRA:
-
Intravenous regional anesthesia
References
Abu MM, Elyazed GM, Eid. (2017) Dexamethasone versus neostigmine as an adjuvant to bupivacaine 0.25% for caudal analgesia in children undergoing open inguinal hernia repair. Egypt Soc Anesth 33:1110–1849
Alam MS, Hoque HW, Saifullah M, Ali MO (2009) Port site and intraperitoneal infiltration of local anesthetics in reduction of postoperative pain after laparoscopic cholecystectomy. Med Today 22(1):24–28
Arora R, Zuberi A, Rastogi B, Singh V, Trivedi A (2019) Comparative evaluation of clinical efficacy of intraperitoneal ropivacaine with and without fentanyl for post-operative analgesia after laparoscopic cholecystectomy. Glob J Res Anal 8(3):5–8
Atia AM, Abdel-Rahman KA (2016) Anesthetic and analgesic effect of neostigmine when added to lidocaine in intravenous regional anesthesia. J Anesth Clin Res 7(660):2
Bisgaard T, Warltier DC (2006) Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the Evidence. Anesthesiology 104:835–846
Bone HG, Van Aken H, Booke M, Bürkle H (1999) Enhancement of axillary brachial plexus block anesthesia by co-administration of neostigmine. Reg Anesth Pain Med 24(5):405–410
Boogmans T, Vertommen J, Valkenborgh T, Devroe S, Roofthooft E, Van de Velde M (2014) Epidural neostigmine and clonidine improves the quality of combined spinal epidural analgesia in labour: a randomised, double-blind controlled trial. Eur J Anaesthesiol 31(4):190–196
Booth JL, Ross VH, Nelson KE, Harris L, Eisenach JC, Pan PH (2017) Epidural neostigmine versus fentanyl to decrease bupivacaine use in patient-controlled epidural analgesia during labor. A randomized, double-blind, controlled study. Anesthesiology 127:50–57
Cossu AP, De Giudici LM, Piras D, Mura P, Scanu M, Cossuc M, Saba M, Finco G, Brazzi L (2015) A systematic review of the effects of adding neostigmine to local anesthetics for neuraxial administration in obstetric anesthesia and analgesia. Int J Obstet Anesthesia 24:237–246
Habib AS, Gan TJ (2006) Use of neostigmine in the management of acute postoperative pain and labour pain: a review. CNS Drugs 20(10):821–839
Hsieh C-Y, Poon Y-Y, Ke T-Y, Chiang M-H, Li Y-Y, Tsai P-N et al (2021) Postoperative vomiting following laparoscopic cholecystectomy is associated with intraoperative fluid administration: a retrospective cohort study. Int J Environ Res Public Health 18(10):5305. https://doi.org/10.3390/ijerph18105305
Mostafa RH, Mekki YMH (2018) Comparative evaluation of intraperitoneal bupivacaine and buivacaine ketamine combined with lung recruitment for reducing postoperative shoulder pain in laparoscopic cholecystectomy. Egypt J Anaesth 34(4):159–164
Petersen PL, Stjernholm P, Kristiansen VB, Torup H, Hansen EG, Mitchell AU, Moeller A, Rosenberg J, Dahl JB, Mathiesen O (2012) The beneficial effect of transversus abdominis plane block after laparoscopic cholecystectomy in day-case surgery: a randomized clinical trial. Anesth Analg 115(3):527–533
Toleska M, Kartalov A, Kuzmanovska B, Panovski M, Shosholcheva M, Dimitrovski A et al (2018) Efficacy of intraperitoneal bupivacaine on pain relief after laparoscopic cholecystectomy. Prilozi 39(1):123–129
Yang LC, Chen LM, Wang CJ, Buerkle H (1998) Postoperative analgesia by intra-articular neostigmine in patients undergoing knee arthroscopy. Anesthesiology 88:334–339
Acknowledgements
Not applicable
Funding
No fund was received for this study.
Author information
Authors and Affiliations
Contributions
MSA was a major contributor in writing the manuscript; SFK analyzed the data; ABE collected the patient data, postoperative hemodynamics, and pain score; and HMA created the study idea. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval from the Kasr Alainy Research Ethics Committee (MS-273-2019) on (1-12-2019) and clinical trial registration (NCT04244097) obtained before performing this study. All patients were agreed to participate in the study and signed an informed written consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arafa, M.S., Ahmed, H.M., Elnabawy, A.B. et al. The evaluation of the analgesic effect of intraperitoneal bupivacaine versus bupivacaine with neostigmine on postoperative pain in laparoscopic cholecystectomy: a randomized controlled double-blinded study. Ain-Shams J Anesthesiol 14, 92 (2022). https://doi.org/10.1186/s42077-022-00289-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00289-0