Abstract
Purpose
This study aimed to evaluate the properties of carrot pomace, including its nutritional composition, physiochemical attributes, change in polyphenols and prebiotic potential during in vitro gastrointestinal digestion and fermentation.
Methods
The study involved evaluating the nutritional and physiochemical properties of carrot pomace. It also assessed an in vitro gastrointestinal digestion model to evaluate changes in polyphenol levels and antioxidant properties at different digestive stages. The study also examined the prebiotic potential by analysing the growth of Lactobacillus acidophilus and short-chain fatty acid production by in vitro fermentation assay. High-resolution liquid chromatography mass spectrometry analysis was used to identify polyphenolic compounds before and after digestion.
Results
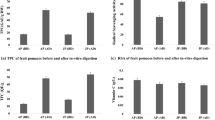
The findings demonstrated that carrot pomace powder contained 43.59% dietary fibre. During in vitro digestion, polyphenol content (total phenolic and flavonoid content) and antioxidant properties (ferric-reducing antioxidant power (FRAP) assay, 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, and 2,2-azinobis-ethylbenzothiozoline-6-sulphonic acid (ABTS) assay) were significantly different at each digestive stage, with strong correlations observed among polyphenols and antioxidant. Notably, polyphenol recovery percentages were significant throughout the digestion phases. Carrot pomace also exhibited prebiotic properties by promoting the growth of L. acidophilus and enhancing short-chain fatty acid production. High-resolution LC–MS analysis revealed eight polyphenolic compounds and their metabolites found in carrot pomace powder (CPP) before and three polyphenolic compounds present after in vitro gastrointestinal digestion.
Conclusion
This research indicated that polyphenols from carrot pomace could potentially play a role in gastrointestinal and colonic health. The valorisation of carrot residues into useful functional ingredient is a novel approach for developing more sustainable food systems as well as the concept of sustainable food.
Graphical Abstract








Similar content being viewed by others
Data availability
Data will be made available on request.
References
Sands RD, Meade B, Seale Jr, et al Scenarios of Global Food Consumption: Implications for Agriculture. 2023, (Report No. ERR-323). U.S. Department of Agriculture, Economic Research Service.
Mirabella N, Castellani V, Sala S. Current options for the valorization of food manufacturing waste: A review. J Clean Prod. 2014;65:28–41. https://doi.org/10.1016/j.jclepro.2013.10.051.
Chiocchio I, Mandrone M, Tomasi P, et al. Plant secondary metabolites: An opportunity for circular economy. Molecules. 2021;26(495):26:495. https://doi.org/10.3390/MOLECULES26020495.
Bellur Nagarajaiah S, Prakash J. Nutritional composition, acceptability, and shelf stability of carrot pomace-incorporated cookies with special reference to total and β-carotene retention. Cogent Food Agri. 2014;1(1):1039886. https://doi.org/10.1080/23311932.2015.1039886.
Dong R, Yu Q, Liao W, et al. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2021;339:127879. https://doi.org/10.1016/j.foodchem.2020.127879.
Zhang G, Chen S, Zhou W, et al. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chem. 2018;269:150–6. https://doi.org/10.1016/j.foodchem.2018.06.132.
Yang I, Jayaprakasha GK, Patil B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct. 2018;9:1235–44. https://doi.org/10.1039/c7fo01749a.
Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: Antioxidants and beyond. Am J Clin Nutr. 2005;81:215S-217S. https://doi.org/10.1093/AJCN/81.1.215S.
Tomas M, Rocchetti G, Ghisoni S, et al. Effect of different soluble dietary fibres on the phenolic profile of blackberry puree subjected to in vitro gastrointestinal digestion and large intestine fermentation. Food Res Int. 2020;130:108954. https://doi.org/10.1016/J.FOODRES.2019.108954.
Swallah MS, Fu H, Sun H, et al. The impact of polyphenol on general nutrient metabolism in the monogastric gastrointestinal tract. J Food Qual. 2020;(2020):1–12. https://doi.org/10.1155/2020/5952834.
Sánchez-Rangel JC, Benavides J, Jacobo-Velázquez DA. Valorization of carrot pomace: UVC induced accumulation of antioxidant phenolic compounds. Appl Sci. 2021;11(22):10951. https://doi.org/10.3390/app112210951.
Negi S. Supply chain efficiency: An insight from fruits and vegetables sector in India. J Oper Supply Chain Manage. 2014;7:154. https://doi.org/10.12660/joscmv7n2p154-167.
de Farias DP, de Araújo FF, Neri-Numa IA, Pastore GM. Prebiotics: Trends in food, health and technological applications. Trends Food Sci Technol. 2019;93:23–35. https://doi.org/10.1016/J.TIFS.2019.09.004.
Kaur R, Panesar PS. Galactooligosaccharides as Potential Prebiotics. Probiotics, prebiotics and synbiotics: Technological advancements towards safety and industrial applications. 2022:272–306. https://doi.org/10.1002/9781119702160.ch12.
Toward R, Montandon S, Walton G, Gibson GR. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes. 2012;3(1):57–60. https://doi.org/10.4161/gmic.19411.
Vazquez‐Olivo G, Gutiérrez‐Grijalva EP, Heredia JB. Prebiotic compounds from agro‐industrial by‐products. J Food Biochem. 2019;43(6):e12711. https://doi.org/10.1111/jfbc.12711.
Salehi F. Recent applications of powdered fruits and vegetables as novel ingredients in biscuits: A review. Nutrire. 2020;45:1–0. https://doi.org/10.1186/s41110-019-0103-8.
Chavan AR, Singh AK, Gupta RK, Nakhate SP, Poddar BJ, Gujar VV, Purohit HJ, Khardenavis AA. Recent trends in the biotechnology of functional non-digestible oligosaccharides with prebiotic potential. Biotechnol Gen Eng Revs. 2023;1:1–46. https://doi.org/10.1080/02648725.2022.2152627.
Xiang Q, Yu Q, Wang H, et al. Immunomodulatory effect of Ganoderma atrum polysaccharides on Th17/Treg balance. J Funct Foods. 2018;45:215–22. https://doi.org/10.1016/j.jff.2018.03.020.
Verspreet J, Damen B, Broekaert WF, et al. A critical look at prebiotics within the dietary fiber concept. Annu Rev Food Sci Technol. 2016;7:167–90. https://doi.org/10.1146/annurev-food-081315-032749.
Medhe S, Anand M, Anal AK. Dietary fibers, dietary peptides and dietary essential fatty acids from food processing by‐products. Food Process By‐Prod Utilizat. 2017;29:111–36. https://doi.org/10.1002/9781118432921.ch6.
Kamiloglu S, Capanoglu E, Bilen FD, et al. Bioaccessibility of polyphenols from plant-processing byproducts of black carrot (Daucus carota L.). J Agricult Food Chem. 2016;30;64(12):2450–8. https://doi.org/10.1021/acs.jafc.5b02640.
Wang D, Williams BA, Ferruzzi MG, D’Arcy BR. Microbial metabolites, but not other phenolics derived from grape seed phenolic extract, are transported through differentiated Caco-2 cell monolayers. Food Chem. 2013;138:1564–73. https://doi.org/10.1016/j.foodchem.2012.09.103.
Gullon B, Pintado ME, Fernández-lópez J. In vitro gastrointestinal digestion of pomegranate peel ( Punica granatum) flour obtained from co-products : Changes in the antioxidant potential and bioactive compounds. J Funct Foods. 2015;19:617–28. https://doi.org/10.1016/j.jff.2015.09.056.
Ajila CM, Rao UP. Mango peel dietary fibre: Composition and associated bound phenolics. J Function Foods. 2013;1;5(1):444–50. https://doi.org/10.1016/j.jff.2012.11.017.
AOAC (2005) Official method of analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24.
Condezo-Hoyos L, Mohanty IP, Noratto GD. Assessing non-digestible compounds in apple cultivars and their potential as modulators of obese faecal microbiota in vitro. Food Chem. 2014;161:208–15. https://doi.org/10.1016/J.FOODCHEM.2014.03.122.
Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–78. https://doi.org/10.1016/S0076-6879(99)99017-1.
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. https://doi.org/10.1016/S0308-8146(98)00102-2.
Gullon B, Pintado ME, Barber X, et al. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res Int. 2015;78:169–76. https://doi.org/10.1016/j.foodres.2015.10.021.
Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. https://doi.org/10.1016/S0076-6879(99)99005-5.
Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5.
Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. https://doi.org/10.1016/S0891-5849(98)00315-3.
Quatrin A, Rampelotto C, Pauletto R, et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J Func Foods. 2020;65:103714. https://doi.org/10.1016/j.jff.2019.103714
Figueroa-Gonzalez I, Rodriguez-Serrano G, Gomez-Ruiz L, Garcia-Garibay M, Cruz-Guerrero A. Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products. Food Sci Technol. 2019;39:747–53. https://doi.org/10.1590/fst.07318.
Jadhao AS, Bhuktar AS. Phytochemical profile of curcuma inodora Blatt. Rhizome extract. Think India J. 2019;22(31):428–35.
Sharma KD, Karki S, Thakur NS, Attri S. Chemical composition, functional properties and processing of carrot—a review. J Food Sci Technol. 2012;49(1):22–32. https://doi.org/10.1007/s13197-011-0310-7
Luca MI, Ungureanu-Iuga M, Mironeasa S. Carrot Pomace Characterization for Application in Cereal-Based Products. Applied Sciences. 2022 Aug 10;12(16):7989.. https://doi.org/10.3390/app12167989
Amin S, Jung S, Kang I, Duval A. Valorization of baby carrot processing waste. J Culinary Sci Technol. 2023;21:1–17. https://doi.org/10.1080/15428052.2021.1879338.
Sharoba AM, Farrag MA, El-Salam A. Utilization of some fruits and vegetables wastes as a source of dietary fibers in cake making. J Food Dairy Sci. 2013;4(9):433–53. https://doi.org/10.21608/JFDS.2013.72084
Šavikin K, Nastić N, Janković T, Bigović D, Miličević B, Vidović S, Menković N, Vladić J. Effect of type and concentration of carrier material on the encapsulation of pomegranate peel using spray drying method. Foods. 2021;10(9):1968. https://doi.org/10.3390/foods10091968
Dong R, Liu S, Zheng Y, et al. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in: In vitro digestion and colonic fermentation. Food Funct. 2020;11:6652–65. https://doi.org/10.1039/d0fo00975j.
Tomas M, Beekwilder J, Hall RD, et al. Effect of dietary fiber (inulin) addition on phenolics and in vitro bioaccessibility of tomato sauce. Food Res Int. 2018;106:129–35. https://doi.org/10.1016/J.FOODRES.2017.12.050.
Catalkaya G, Venema K, Lucini L, et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020;1:109–33. https://doi.org/10.1002/FFT2.25.
Juániz I, Ludwig IA, Bresciani L, et al. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J Funct Foods. 2017;32:195–207. https://doi.org/10.1016/J.JFF.2017.02.033.
Padayachee A, Netzel G, Netzel M, et al. Binding of polyphenols to plant cell wall analogues—part 2: Phenolic acids. Food Chem. 2012;135:2287–92. https://doi.org/10.1016/J.FOODCHEM.2012.07.004.
Fernandes A, Mateus N, de Freitas V. Polyphenol-dietary fiber conjugates from fruits and vegetables: nature and biological fate in a food and nutrition perspective. Foods. 2023;12(5):1052. https://doi.org/10.3390/foods12051052.
Martínez-Las Heras R, Pinazo A, Heredia A, Andrés A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017;214:478–85. https://doi.org/10.1016/J.FOODCHEM.2016.07.104.
Bas-Bellver C, Andrés C, Seguí L, et al. Valorization of persimmon and blueberry byproducts to obtain functional powders: in vitro digestion and fermentation by gut microbiota. J Agric Food Chem. 2020;68:8080–90. https://doi.org/10.1021/ACS.JAFC.0C02088.
Bas-Bellver C, Barrera C, Betoret N, Seguí L. Effect of processing and in vitro digestion on bioactive constituents of powdered IV range carrot (Daucus carota, L.) wastes. Foods. 2023;12(4):731. https://doi.org/10.3390/foods12040731.
Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011;128:14–21. https://doi.org/10.1016/J.FOODCHEM.2011.02.052.
Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–74. https://doi.org/10.1016/J.FOODCHEM.2006.06.025.
Lucas-Gonzalez R, Navarro-Coves S, Pérez-Álvarez JA, et al. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind Crops Prod. 2016;94:774–82. https://doi.org/10.1016/j.indcrop.2016.09.057.
Alminger M, Aura AM, Bohn T, et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr Rev Food Sci Food Saf. 2014;13:413–36. https://doi.org/10.1111/1541-4337.12081.
Xiao P, Huang H, Chen J, Li X. In vitro antioxidant and anti-inflammatory activities of Radix Isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J Ethnopharmacol. 2014;157:55–61. https://doi.org/10.1016/J.JEP.2014.09.005.
Mosele JI, Macià A, Romero MP, et al. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J Funct Foods. 2015;14:529–40. https://doi.org/10.1016/J.JFF.2015.02.026.
He Z, Tao Y, Zeng M, et al. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016;200:107–16. https://doi.org/10.1016/J.FOODCHEM.2016.01.045.
Dong R, Liu S, Xie J, Chen Y, Zheng Y, Zhang X, Zhao E, Wang Z, Xu H, Yu Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res Int. 2021;143:110263. https://doi.org/10.1016/j.foodres.2021.110263.
Chandrasekara A, Shahidi F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Func Foods. 2012;4(1):226–37. https://doi.org/10.1016/j.jff.2011.11.001.
Liu S, Jia M, Chen J, et al. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019;93:284–92.
Topakas E, Vafiadi C, Christakopoulos P. Microbial production, characterization and applications of feruloyl esterases. Process Biochem. 2007;42:497–509. https://doi.org/10.1016/J.PROCBIO.2007.01.007.
Requena T, Monagas M, Pozo-Bayón MA, et al. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Sci Technol. 2010;21:332–44. https://doi.org/10.1016/J.TIFS.2010.04.004.
Holgado F, Campos-Monfort G, de las Heras C, Rupérez P. In vitro fermentability of globe artichoke by-product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact Carbohydr Diet Fibre. 2021;26:100286. https://doi.org/10.1016/j.bcdf.2021.100286.
Huebner J, Wehling RL, Hutkins RW. Functional activity of commercial prebiotics. Int Dairy J. 2007;17:770–5. https://doi.org/10.1016/J.IDAIRYJ.2006.10.006.
Hotchkiss AT, Chau HK, Strahan GD, et al. Carrot rhamnogalacturonan I structure and composition changed during 2017 in California. Food Hydrocoll. 2023;137:108411. https://doi.org/10.1016/J.FOODHYD.2022.108411.
Flint HJ, Bayer EA, Rincon MT, et al. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31.
Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–59. https://doi.org/10.1111/J.1365-2672.1991.TB02739.X.
da Silva JK, Cazarin CB, Junior SB, Augusto F, Junior MR. Passion fruit (Passiflora edulis) peel increases colonic production of short-chain fatty acids in Wistar rats. LWT-Food Sci Technol. 2014;59(2):1252–7. https://doi.org/10.1016/j.lwt.2014.05.030.
Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition. 2010;26:653–61. https://doi.org/10.1016/J.NUT.2009.07.006.
Fung KYC, Cosgrove L, Lockett T, et al. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108:820–31. https://doi.org/10.1017/S0007114512001948.
De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. https://doi.org/10.1016/J.CELL.2013.12.016.
Jakobsdottir G, Nyman M, Fåk F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition. 2014;30:497–502. https://doi.org/10.1016/J.NUT.2013.08.013.
Tingirikari JMR. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018;9:5059–73. https://doi.org/10.1039/C8FO01296B.
Sleeth ML, Thompson EL, Ford HE, Zac-Varghese SE, Frost G. Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutrit Res Rev. 2010;23(1):135–45. https://doi.org/10.1017/S0954422410000089.
Ortega N, Macià A, Romero MP, et al. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011;124:65–71. https://doi.org/10.1016/J.FOODCHEM.2010.05.105.
Liang A, Leonard W, Beasley JT, et al. Anthocyanins-gut microbiota-health axis: a review. Crit Rev Food Sci Nutrit . 2023;9:1–26. https://doi.org/10.1080/10408398.2023.2187212.
Acknowledgements
The authors would like to thank SHODH-Scheme Developing High Quality Research, Knowledge Consortium of Gujarat, Gujarat Education Department, Ahmedabad, Gujarat India, for providing SHODH fellowship.
Author information
Authors and Affiliations
Contributions
Urvashi P. Mall: investigation, formal analysis, methodology and writing—original draft. V. H. Patel: conceptualisation, supervision and writing—review and editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mall, U.P., Patel, V.H. Carrot pomace powder: a promising source of polyphenols and prebiotics for improving gut health. Nutrire 49, 9 (2024). https://doi.org/10.1186/s41110-023-00250-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-023-00250-7