- Original article
- Open access
- Published:
Isolation of a bacteriophage targeting Pseudomonas aeruginosa and exhibits a promising in vivo efficacy
AMB Express volume 13, Article number: 79 (2023)
Abstract
Pseudomonas aeruginosa is an important pathogen that causes serious infections. Bacterial biofilms are highly resistant and render bacterial treatment very difficult, therefore necessitates alternative antibacterial strategies. Phage therapy has been recently regarded as a potential therapeutic option for treatment of bacterial infections. In the current study, a novel podovirus vB_PaeP_PS28 has been isolated from sewage with higher lytic activity against P. aeruginosa. Isolated phage exhibits a short latent period, large burst size and higher stability over a wide range of temperatures and pH. The genome of vB_PaeP_PS28 consists of 72,283 bp circular double-stranded DNA, with G + C content of 54.75%. The phage genome contains 94 open reading frames (ORFs); 32 for known functional proteins and 62 for hypothetical proteins and no tRNA genes. The phage vB_PaeP_PS28 effectively inhibited the growth of P. aeruginosa planktonic cells and displayed a higher biofilm degrading capability. Moreover, therapeutic efficacy of isolated phage was evaluated in vivo using mice infection model. Interestingly, survival of mice infected with P. aeruginosa was significantly enhanced upon treatment with vB_PaeP_PS28. Furthermore, the bacterial load in liver and kidney isolated from mice infected with P. aeruginosa and treated with phage markedly decreased as compared with phage-untreated P. aeruginosa-infected mice. These findings support the efficacy of isolated phage vB_PaeP_PS28 in reducing P. aeruginosa colonization and pathogenesis in host. Importantly, the isolated phage vB_PaeP_PS28 could be applied alone or as combination therapy with other lytic phages as phage cocktail therapy or with antibiotics to limit infections caused by P. aeruginosa.
Introduction
Pseudomonas aeruginosa is a causative agent of wide variety of infections ranging from soft tissue infections to life-threatening ones including bacteremia and pneumonia. Furthermore, Pseudomonas aeruginosa is a global opportunistic pathogen and a major cause of nosocomial infections due to the flexibility and adaptability encoded in its genome (Gellatly and Hancock 2013). P. aeruginosa could grow on different medical equipment due to the presence of its essential binding factors such as flagella, pili and the ability to form biofilms (Remold et al. 2011; Gale et al. 2015). Unfortunately, infections caused by this bacterium are characterized by higher morbidity and mortality rates especially in immunocompromised patients and those suffering from severe burns or cystic fibrosis (Guarner and Malagelada 2003; English and Gaur 2010).
Numerous virulence factors contribute to P. aeruginosa pathogenesis including hemolysins, rhamnolipids, proteases and biofilms (Lee and Zhang 2015). P. aeruginosa is capable of forming biofilms which protect bacteria from environmental stresses and phagocytosis and lead to long-term persistence (Moradali et al. 2017). Moreover, P. aeruginosa is able to acquire resistance through mutation and horizontal gene transfer added to its intrinsic resistance to various antibiotics including beta-lactams (Fajardo et al. 2008; Breidenstein et al. 2011). Therefore, treatment of P. aeruginosa infections by conventional antibiotics has become a major global challenge due to bacterial resistance.
Recently, due to increased antimicrobial resistance, there is an urgent necessity for the development of alternative antimicrobial approaches to efficiently control bacterial infections (Cegelski et al. 2008). Bacteriophages (phages) have been considered as a potential therapeutic option due to their safety and avoidance of harm to normal flora (Taati Moghadam et al. 2020). Meanwhile, antibiotic resistance increases, phages retain its ability to compact antibiotic-resistant bacteria in addition to its ability to inhibit biofilms (Abedon et al. 2017; Taati et al. 2020). Furthermore, there are no obvious adverse effects associated with phage therapy due to the higher phage specificity towards target bacterial species without affecting the host microbiota (Skurnik et al. 2007). Phages have valuable properties such as they are easy to culture, economic and can be stored for long periods (Pohane et al. 2014). Furthermore, endotoxin released upon lysis of bacterial cells following treatment with phages is relatively lower compared to that released by antibiotics (Dufour et al. 2017). Therefore, phage therapy is believed to be a promising tool for management of bacterial infections and therefore could be considered for treatment of P. aeruginosa infections alone or in combination with antibiotics (Krylov et al. 2015; Thanh et al. 2020).
While a lot of previous studies have isolated several phages infecting P. aeruginosa, many of these studies suffer from serious gaps regarding detailed characterization of isolated phages. For instance, the genomes of some previously isolated phages have not been fully characterized (Kumari et al. 2009; Azizian et al. 2015; Didamony et al. 2015; Barazandeh et al. 2021). Furthermore, neither the antibiofilm potential of isolated phages nor their in vivo antibacterial efficacy has been investigated in these studies (Miyata et al. 2014; Cao et al. 2015; Shigehisa et al. 2016; Tang et al. 2018; de Melo et al. 2019; Alvi et al. 2020; Enwuru et al. 2021; Namonyo et al. 2022). Therefore, complete genome analysis, the antibiofilm activity and the ability of isolated phage to reduce P. aeruginosa pathogenesis in vivo will be fully covered herein. The current study aims to isolate and characterize a virulent phage targeting P. aeruginosa isolated from various clinical sources. The physical properties, antibiofilm activity as well as whole genome sequencing of isolated phage will be determined herein. Moreover, the influence of isolated phage on P. aeruginosa pathogenesis in host will be assessed in vivo using mice infection model. The findings of present study would be of great importance and helpful in treatment of P. aeruginosa related infections.
Material and methods
Isolation and identification of P. aeruginosa
Clinical P. aeruginosa isolates were provided by the clinical laboratories of Zagazig University Hospitals, Zagazig, Egypt with no direct involvement of patients in the study. P. aeruginosa isolates were further identified biochemically according to Douraghi et al. (2014). In addition to clinical isolates, P. aeruginosa reference strains; ATCC 27853, ATCC 9027 and PAO1 were included in this study. Bacterial strains were stored in Muller Hinton (MH) broth containing 20% glycerol and kept at − 80 °C. The bacterial strains used in current study are listed in (Additional file 1: Table S1).
Antimicrobial susceptibility testing
The susceptibility of P. aeruginosa strains to different antibiotics was determined by Kirby-Bauer standard disc diffusion method (Patel et al. 2015). Diameters of inhibition zones were measured and bacterial susceptibility to antibiotics was interpreted as resistant (R), intermediate (I) and susceptible (S) according to guidelines recommended by CLSI, (2018) (Humphries et al. 2018).
Quantitative assessment of biofilm formation
The capacity of P. aeruginosa to form biofilm was assayed spectrophotometrically as previously reported by Stepanović et al. (2007). In brief, bacterial suspensions were allowed to form biofilms in 96-well polystyrene U-shaped microtiter plate (Costar™; Corning™) and incubated for 24 h at 37 °C. Wells contained tryptone soya (TS) broth only were included as negative control. Fixed biofilms were stained by using 1% crystal violet and bound dye was dissolved by 33% glacial acetic acid. The optical densities were measured spectrophotometrically at 570 nm (Bio-Tek synergy HT microplate reader, USA). The cut-off optical density (ODc) was calculated as three times standard deviations above the mean OD of the negative control. Bacterial isolates were categorized based on biofilm forming capacity following the criteria mentioned before (Stepanović et al. 2007).
Bacteriophage isolation
The phage was isolated from sewage by the enrichment technique (Didamony et al. 2015; Chen et al. 2021). The sample was clarified through centrifugation at 6000×g for 20 min and filtered through a 0.45 μm membrane filter (Millipore, USA). The filtrate was added to an equal volume of double concentrated TS broth medium containing exponential phase culture of P. aeruginosa (PS28) as a host strain. This bacterial strain was isolated from patient suffering from urinary tract infection. Suspensions were incubated in shaker incubator at 37 ºC for 24 h. The cultures were centrifuged at 6000×g for 10 min and the supernatants were filtered through 0.22 μm membrane filter to remove bacteria. The filtrates were checked for presence of bacteriophages by the spot assay. Furthermore, the phage presence was characterized by the double agar layer method as described before (Mazzocco et al. 2009). Briefly, a mixture composed of 3 mL of prewarmed soft TS agar (0.6% agar) and 100 µL of P. aeruginosa culture grown to the exponential phase were poured over solid bottom TS agar plate. After solidifying, 10 μL of filtered suspension was spotted onto bacterial lawns then left to dry and incubated overnight at 37 °C. Appearance of clear zone (plaques) in the plate indicates presence of bacteriophages.
Bacteriophage purification and propagation
Bacteriophage purification was done by picking a well isolated single plaque and resuspended in SM buffer [100 mM NaCl, 8 mM MgSO4, 50 mM Tris–HCl (pH 7.4), 0.01% gelatin]. Then, aliquots of 100 μL of serial diluted phage was mixed with 100 μL of P. aeruginosa culture (PS28) and plated by soft agar overlay technique. This process was performed successive rounds in order to obtain uniform plaque morphology. Phage propagation was done by incubating the phage with P. aeruginosa (PS28) as host with shaking at 120 rpm for 24 h. The culture was centrifuged and the supernatant was filtered. The phage was propagated to obtain a high titer stock as described (Kumari et al. 2009) and purified phage stock was stored at 4 °C (Russell and Sambrook 2001).
Transmission electron microscopy (TEM)
The phage morphology was visualized using the transmission electron microscope (TEM) as described (Shen et al. 2016). A drop of high titer purified phage [1012 particle forming unit (PFU)/mL] was applied to carbon-coated copper grid (200 mesh). Phage particles were negatively stained with 2% phosphotungstic acid (pH 7). Finally, the grid was air-dried and phage particles were examined using TEM (Hitachi H600A, Japan).
Determination of phage host range and efficiency of plating (EOP)
The host range of isolated phage against a total of 18 P. aeruginosa strains (15 P. aeruginosa isolates from different clinical sources and 3 P. aeruginosa reference strains; P. aeruginosa ATCC 27853, P. aeruginosa ATCC 9027 and P. aeruginosa PAO1) in addition to other bacterial species (Escherichia coli, Salmonella Typhimurium, Klebsiella pneumoniae, Serratia marcescens and Staphylococcus aureus) was performed using the spot testing method and as described above. The selected clinical P. aeruginosa isolates were chosen to be representative for different clinical sources including; burn and urine (4 isolates each), wound and endotracheal aspirates (3 isolates each) and ear infections (1 isolate) in order to give a full picture about susceptibility of P. aeruginosa isolates from various clinical sources to infection with isolated phage. Clear inhibition zone was considered as evidence for bacterial susceptibility to phage (Adnan et al. 2020). The efficiency of plating (EOP) of isolated phage was evaluated against P. aeruginosa isolates that showed lysis in the spot assay. Aliqutoes of 100 µL of bacterial cultures grown to the exponential phase were co-cultured with 100 µL of tenfold serially diluted phage in soft agar layer and overlayed on surface of TS agar plates. The plates were incubated overnight at 37 °C and the PFUs were counted for each phage-bacterium combination. The EOP values were estimated by dividing the total number of PFUs obtained by the target bacteria to the total number of PFUs obtained by host bacteria; P. aeruginosa PS28. Assays were repeated three times and results were recorded according as the follow; High production if the EOP ratio was ≥ 0.5; Medium production if 0.5 > EOP ratio ≥ 0.1; Low production if 0.1 > EOP > 0.001 and inefficient if EOP ≤ 0.001 (Khan Mirzaei and Nilsson 2015).
Temperature and pH stability
For thermal stability evaluation, aliquot of 100 µL of purified phage particles was mixed with 900 µL of SM buffer and placed in an adjusted water bath incubator at various temperatures (4, 40, 50, 60, 70, 80, 90 and 100 °C) for 1 h. The phage titer was determined after incubation at each specified temperature by the double layer agar technique. Similarly, the impact of pH on phage survival was assessed. Purified phage particles were incubated for 1 h in SM buffer at different pH (3, 4, 5, 6, 7, 8, 9, 10, 11 and 12) adjusted using either 1 M HCL or 1 M NaOH followed by determining the phage titer. These assays were carried out in triplicate and the phage titers were estimated as described before (Asif et al. 2020).
One-step growth curve
The one-step growth curve was performed as previously described to determine the phage growth features (Cao et al. 2015). Briefly, 9 mL of host bacterium culture; P. aeruginosa PS28 was incubated with 90 µL of phage suspension at multiplicity of infection (MOI) of 0.1 (107 PFU/mL) for 10 min to allow phage adsorption. Next, the mixture was centrifugated at 10,000 ×g for 10 min and the pellet was resuspended in 10 mL TS broth. Samples of 100 µL were collected at time intervals of 5 min over and subjected to phage titration by the double layer agar method. The assay was evaluated in triplicate and both phage latent period and burst size were determined. The phage burst size was determined as the ratio of the average number of free phage particles after the release phase to their number during the latency phase (Cao et al. 2015).
In vitro killing assay
The lytic activity of isolated phage against both the host strain (PS28) and PAO1, a strain with high EOP ratio, was assayed. Phage suspensions at different MOIs (0.1, 1 and 10) were co-cultured with 108 CFU/mL of bacterial suspensions and incubated with shaking at 37 °C. The inhibitory effect of isolated phage on bacterial growth was determined spectrophotometrically at OD600 and compared with bacterial culture without phage. Furthermore, the number of revival bacteria and phage were counted following phage infection (Morozova et al. 2022). Briefly, phage suspensions at different MOIs (0.1, 1 and 10) were co-cultured with bacterial suspensions and incubated overnight with shaking at 37 °C. Then, aliquots were taken, serially diluted and plated over TS agar plates. The assays were performed in triplicate and results were expressed as means ± standard errors (Chen et al. 2018).
Biofilm inhibition assay
The ability of isolated phage to eradicate biofilms formed by P. aeruginosa was characterized (Liu et al. 2020). Bacterial cultures were allowed to form biofilm onto the surface of 96-well polystyrene U-shaped microtiter plate (Costar™; Corning™) exactly as described above. Following incubation, broth culture was gently decanted and wells were washed with sterile phosphate buffer saline (PBS) to remove the planktonic cells. Next, aliquotes of about 200 μL of phage suspension at various MOIs (0.1, 1 and 10) in TS broth were added to each well and incubated overnight at 37 °C. Control wells received sterile TS broth only without phage. The formed biofilms were assayed using the crystal violet assay and absorbance was measured spectrophotometrically at 570 nm. The experiment was performed in triplicate and results were expressed as means ± standard errors.
Bacteriophage genome sequencing and data analysis
The phage vB_PaeP_PS28 nucleic acid was extracted from phage lysate (2.5 × 1012 PFU/mL) using QIAamp1 DNA Mini kit (QIAGEN, Germany) following the manufacturer guidelines and DNA pellet was stored at − 20 °C until use. The DNA library was prepared using the Nextera XT DNA Library preparation kit (Illumina, USA). The DNA was fragmented then tagged utilizing the transposome in the Nextera XT Kit. The whole genome sequencing was done by Illumina Miseq next-generation sequencing at Genomics and Epigenomics Program, Children’s Cancer Hospital Egypt, Cairo, Egypt. All preparations and the sequencing run were performed according to Illumina manufacturing instructions. The quality of paired-end DNA reads was evaluated using FASTQC (Brown et al. 2017). Moreover, low-quality bases were trimmed using Trimmomatic v0.36. The generated sequences were assembled using Unicycler v0.4.8 to assemble the reads into contigs (Wick et al. 2017). Assembly quality was checked using QUAST v 5.0.2 (Gurevich and Saveliev 2013). The number of open reading frames (ORFs) in phage genome was predicted and putative functions of predicted ORFs were annotated using Prokka v 1.14 (Seemann 2014). The circular genomic map of vB_PaeP_PS28 was generated and visualized via CGView (Stothard and Wishart 2005). The online tool tRNAscan-SE 1.21 was used to look for tRNA genes in the phage sequences (Chan and Lowe 2019). The phylogeny tree was generated using BLAST (Basic Local Alignment Search Tool) using the neighbor-joining method. Intergenomic similarity between isolated phage and other related Pseudomonas phages was calculated using VIRIDIC v1.1; the Virus Intergenomic Distance Calculator (Moraru et al. 2020). In addition, phylogenetic trees based on the terminase large subunit and RNA polymerase large subunit were constructed (Altschul et al. 1990). The Dot Plot analysis was performed using VectorBuilder’s Sequence Dot Plot tool and comparative analysis of the whole genome with other related phages was performed by Easyfig program (Sullivan et al. 2011).
Assessment of the bacteriolytic activity of isolated phage in vivo using mice infection model
The influence of isolated phage on P. aeruginosa pathogenesis was characterized in vivo using mice infection model (Alvi et al. 2021). All procedures in animal infection experiment were performed according to ethical standards of the Zagazig University Institutional Animal and Use Committee (ZU-IACUC), which was granted Approval Number (ZU-IACUC/3/F/72/2022). Briefly, five groups (15 mice each; 6 mice for survival experiment and 9 mice for determining phage and bacterial load) of 4 weeks old albino mice were included in the experiment. The first group contained mice inoculated intraperitoneally (I.P) with P. aeruginosa at (2.5 × 107 CFU/mL), the second group contained Pseudomonas-inoculated mice and treated intraperitoneally with isolated phage at MOI = 100 (2.5 × 109 PFU/mL). The third group contained mice inoculated with isolated phage (2.5 × 109 PFU/mL) only. In addition, both non-injected and PBS-injected mice groups were included as controls. Mice survival in each group was monitored daily over 4 days-period and plotted using Kaplan–Meier method using Log-rank test for statistical analysis. In addition to mice survival, three mice from each group were anesthetized and sacrificed at 24, 48 and 72 h post inoculation. Mice liver and spleen were aseptically obtained for determination of both bacterial burden and phage titer. Isolated organs were homogenized, serially diluted in PBS and plated on cetrimide agar plates for enumeration of bacterial CFUs. In addition, the homogenate was filtered, serially diluted and overlaid by the double layer agar method to determine the phage titer in treated mice and expressed as (PFUs). Both bacterial load and phage titer were determined and expressed as means ± standard errors. The statistical analysis was performed by Mann–Whitney U analysis with P < 0.05 was considered significant.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software using Student t-tests or one-way ANOVA unless otherwise stated. All experiments were performed in triplicate and data expressed as the mean ± standard errors.
Results
Antibiotic susceptibility and assessment of biofilm formation by P. aeruginosa isolates
A total of 50 P. aeruginosa isolates obtained from different clinical sources; burns (10 isolates), surgical wounds (10 isolates), urine (11 isolates), ear infections (2 isolates) and endotracheal aspirates (17 isolates) were included in this study. The susceptibility profile of P. aeruginosa isolates against different antibiotics was determined by the disc diffusion method and results were interpreted according to CLSI (2018) guidelines (Additional file 1: Table S1). All P. aeruginosa isolates were sensitive to colistin while high bacterial resistance was observed towards gentamicin (60%). Majority of P. aeruginosa isolates exhibited high resistance to fluoroquinolone and carbapenems (58% and 54%; each). The antibiotic susceptibility results reveal that about of 56% of P. aeruginosa isolates were multi-drug resistant (MDR). In addition to antibiotic susceptibility, the biofilm forming capacity of P. aeruginosa isolates was assessed spectrophotometrically by the crystal violet assay. Bacterial isolates were categorized into 3 groups according to biofilm formation; strong (20.4% of bacterial isolates), moderate (64.8% of bacterial isolates) and weak biofilm forming (14.8% of bacterial isolates) as shown in (Additional file 1: Fig. S1).
Phage isolation
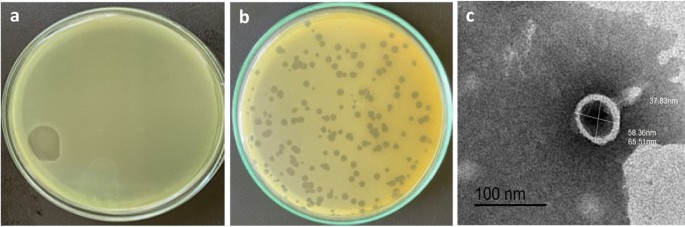
A lytic phage specific for P. aeruginosa was isolated from sewage by the enrichment technique using P. aeruginosa PS28 as host strain. P. aeruginosa PS28 was found to be MDR strain and exhibit a strong biofilm-forming capability. The isolated phage was designated as vB_PaeP_PS28 according to the recommended nomenclature procedure (Kropinski et al. 2009). P. aeruginosa phage vB_PaeP_PS28 produced circular plaques with diameter of 2–3 mm in double layer agar method (Fig. 1a, b). Plaques of homogenous morphology were selected, purified several rounds for further analysis and purified phage stock stored in SM buffer at 4 °C.
Isolation of bacteriophage. a Clear lytic zone on bacterial lawn by spot assay of phage lysate from sewage. b Plaque morphology of isolated phage double layer agar plate. c Transmission electron microscope (TEM) images of vB_PaeP_PS28. Phage particles were negatively stained by 2% phosphotungstic acid. Scale bar = 100 nm
Phage morphology characterization by transmission electron microscopy (TEM)
TEM images revealed that the phage vB_PaeP_PS28 possesses an icosahedral head and short non-contractile tail which are closely related to phages belonging to Podoviridae family according to International Committee Taxonomy of Viruses (ICTV) (Adriaenssens and Brister 2017). The phage head diameter is 65.5 nm and tail length of 37.8 nm that are extremely typical to the Podoviridae family within the order Caudovirales (Fig. 1c).
Host range and efficiency of plating (EOP) of the phage vB_PaeP_PS28
The bacteriolytic activity of vB_PaeP_PS28 against different P. aeruginosa strains and other bacterial species was evaluated by the spot assay. The phage vB_PaeP_PS28 has the ability to infect and lyse approximately 13/18 (72.2%) of tested P. aeruginosa strains which indicates a broad spectrum lytic activity of isolated phage (Table 1). Moreover, the phage vB_PaeP_PS28 able to infect most of tested MDR P. aeruginosa strains (8/9) from different clinical sources such as burn, wound, urine, ear infections and endotracheal aspirates (Additional file 1: Table S2). However, no lytic activity was observed against other bacterial species by phage vB_PaeP_PS28. Furthermore, the susceptibility of tested isolates to the phage vB_PaeP_PS28 was confirmed by EOP analysis. Series of diluted phage vB_PaeP_PS28 were plated against susceptible strains. EOP values of phage-bacteria mixtures were varied into low (n = 3); medium (n = 4) and high EOP values (n = 6) (Table 1).
Temperature and pH stability of the phage vB_PaeP_PS28
The thermal stability of vB_PaeP_PS28 was assessed by monitoring change in phage titer upon incubation under different temperatures. The results indicate that the phage vB_PaeP_PS28 is tolerant to wide range of temperatures. Isolated phage could survive up to 60 °C with no significant reduction in phage titer. However, there was a significant reduction in phage titer upon incubation of vB_PaeP_PS28 at higher temperatures (70, 80, 90 and 100 °C) as shown in Fig. 2a. Regarding phage pH stability, the phage vB_PaeP_PS28 was able to retain its infectivity and could survive at pH ranges from 4 to 10. There was a slight reduction in phage viability at pH 11 and viable phage particles were not observed at extreme pH (3 and 12) (Fig. 2b).
One-step growth curve and in vitro killing assay
The one-step growth curve revealed that the phage vB_PaeP_PS28 has a latent period of 15 min and an average burst size of 210 virions per infected bacterium (Fig. 3). The bacteriolytic activity of vB_PaeP_PS28 at different MOIs (0.1, 1 and 10) was determined against both the phage host strain PS28 and PAO1. As shown in Fig. 4, control culture without phage treatment continued to grow during the incubation period. On the other hand, the phage vB_PaeP_PS28 was able to adversely affect bacterial growth over 24 h. Importantly, bacterial growth inhibition was found to be dose dependent where growth inhibition was higher at MOI of 10 as compared to MOI of 1 and 0.1. In addition, the vB_PaeP_PS28 phage could effectively reduce the number of surviving bacterial count in MOI dependent manner (Additional file 1: Table S3). Thus, the obtained data demonstrated high lytic activity of isolated phage.
One-step growth curve of vB_PaeP_PS28. Phage was incubated with exponential culture of PS28 for 10 min, centrifuged and pellet was resuspended in TS broth. Titer of free phages was determined by double layer agar technique. Three biological replicates were performed and data were presented as mean ± SE
Bacteriolytic activity of phage vB_PaeP_PS28 against the host strain P. aeruginosa PS28 (a) and PAO1 b. Early exponential bacterial cultures were incubated with and without isolated phage suspension at MOI of (0.1, 1 and 10) at 37 °C for 24 h. Bacterial growth was determined and measured spectrophotometrically at OD600. The results were expressed as means ± SE of three independent experiments
Biofilm inhibition assay
The antibiofilm activity of vB_PaeP_PS28 against five strong biofilm forming clinical isolates including its host strain PS28 as well as P. aeruginosa ATCC 9027 and ATCC 27853 was assessed by the crystal violet assay. The phage vB_PaeP_PS28 effectively degraded mature biofilms and reduced biofilm biomass formed by all tested P. aeruginosa strains. As shown in Fig. 5, the antibiofilm activity of vB_PaeP_PS28 against P. aeruginosa was MOI dependent where maximum biofilm inhibition was observed at MOI of 10 as compared with MOI of 1 and 0.1.
The antibiofilm activity of vB_PaeP_PS28 against various P. aeruginosa isolates. Biofilms were formed in 96-well plates for 24 h and treated with phage at different MOIs (0.1, 1 and 10) for 24 h. Formed biofilms were stained by 1% crystal violet and measured spectrophotometrically at OD600. The experiment was carried out at three independent replicates and data was expressed as means ± SE with P < 0.05 was considered significant
Phage genome features
The whole genome of vB_PaeP_PS28 was sequenced by Illumina Miseq and assembly was performed by Unicycler v0.4.8. The phage vB_PaeP_PS28 genome is composed of 72,283 bp of circular double-stranded DNA, with 54.75% G + C content. The entire genome structure of vB_PaeP_PS28 is shown in Fig. 6a. In total, 94 ORFs were found, including only 32 ORFs (34%) were predicted to encode for functional proteins, whereas 62 ORFs (66%) were annotated as hypothetical proteins. The predicted functions of 32 ORFs were divided into 5 major modules including structure proteins (ORF 8, ORF 9, ORF 18, ORF 21, ORF 24, ORF 88, ORF 90 and ORF 93), DNA metabolism and replication (ORF 1, ORF 11, ORF 34, ORF 35, ORF 37, ORF 52, ORF 56, ORF 59, ORF 69, ORF 71 and ORF 84), packaging and assembly proteins (ORF 27, ORF 32, ORF 81, ORF 82, ORF 83 and ORF 85), host cell lysis modules (ORF 25, ORF 38, ORF 42 and ORF 51) and additional functions (ORF 30, ORF 31 and ORF 40) (Table 2). ORFs 30 and 31 play a role in cell lysis inhibition and interference with cell metabolism. This would delay releasing of phage holin enzyme until the phage particles are formed and accumulated within the host. On the other hand, ORF 40 is involved in energy-requiring activities such as phage DNA packaging replication. The phage genome did not reveal any lysogenic genes or host genome-related sequences, so that vB_PaeP_PS28 referred to be a lytic phage. The genes related to antibiotic resistance in P. aeruginosa, host virulence factors and toxin genes were also absent in vB_PaeP_PS28 phage genome. The phylogenetic analysis (Fig. 6b) shows that vB_PaeP_PS28 is closely related to Pseudomonas phage vB_PaeP_FBPa1 (GenBank Acc. No ON857943.1), which is a member of the family Podoviridae and the genus Litunavirus. Additionally, phylogenetic trees were created for some of the predicted essential phage proteins; the terminase large subunit (Fig. 6c) and RNA polymerase large subunit (Fig. 6d). Importantly, the genomic sequence similarity of the phage vB_PaeP_PS28 to other previously characterized phages infecting P. aeruginosa was determined. As shown in (Table 3, Fig. 7 and Additional file 1: Fig. S2), greatest similarity was observed between the phage vB_PaeP_PS28 and Pseudomonas phage vB_PaeP_FBPa1 (GenBank Acc. No. ON857943.1, identity, 94.81%); Pseudomonas phage VB_PaeS_VL1 (GenBank Acc. No. OK665488.1, identity, 94.37%); Pseudomonas phage YH6 (GenBank Acc. No. KM974184.1, identity, 94.07%); and Pseudomonas phage PA26 (GenBank Acc. No. NC_041907.1, identity, 94.04%). To further examine the taxonomy of the vB_PaeP_PS28 phage, matrix of intergenomic similarities of the vB_PaeP_PS28 genome with four most similar phage genomes Pseudomonas phage vB_PaeP_FBPa1, Pseudomonas phage VB_PaeS_VL1, Pseudomonas phage YH6 and Pseudomonas phage PA26 was calculated using VIRIDIC (Fig. 8). The genome sequence of the phage vB_PaeP_PS28 has been deposited in the GenBank database under GenBank Acc. No. OQ134474.
Genomic characterization of vB_PaeP_PS28. a Circular genomic map of vB_PaeP_PS28; from inside to outside, the first to third circles represent the scale, GC Skew, and GC content respectively; the fourth represents the position of ORFs. The prediction and direction of ORFs are indicated by arrow heads. The genomic map was generated and visualized using CGView b Phylogenetic analysis of vB_PaeP_PS28 and other closely related sequences. c Phylogenetic tree analysis based on the amino acid sequence of terminase large subunit. d Phylogenetic tree analysis based on the amino acid sequence of RNA polymerase large subunit. Phylogenetic trees were constructed using BLAST (Basic Local Alignment Search Tool) using neighbor-joining method
Comparative genomic analysis between phage vB_PaeP_PS28 and related sequences. Pseudomonas phage vB_PaeP_FBPa1 (GenBank Acc. No. ON857943.1), Pseudomonas phage VB_PaeS_VL1 (GenBank Acc. No. OK665488.1), Pseudomonas phage YH6 (GenBank Acc. No. KM974184.1) and Pseudomonas phage PA26 (GenBank Acc. No. NC_041907.1). Sequence similarity is represented by the gray scale bar. The coding sequences are represented by directional arrows. Predicted ORFs in vB_PaeP_PS28 genome are listed below. Comparative analysis was performed using Easyfig
VIRIDIC heatmap of vB_PaeP_PS28 phage and its closest homologues. Intergenomic similarities between the genomic nucleotide sequences of vB_PaeP_PS28 and related bacteriophages infecting P. aeruginosa; Pseudomonas phage vB_PaeP_FBPa1 (GenBank Acc. No. ON857943.1), Pseudomonas phage YH6 (GenBank Acc. No. KM974184.1), Pseudomonas phage PA26 (GenBank Acc. No. NC_041907.1) and Pseudomonas phage VB_PaeS_VL1 (GenBank Acc. No. OK665488.1). Color coding scales are represented above the matrix with intensity of color corresponding to level of similarity
In vivo characterization of the influence of phage vB_PaeP_PS28 on P. aeruginosa pathogenesis
The effect of phage vB_PaeP_PS28 on P. aeruginosa pathogenesis was evaluated in vivo using mice infection model. Mice survival as well as both bacterial and phage counts were monitored in infected mice to evaluate whether vB_PaeP_PS28 phage possesses a protective effect in vivo against P. aeruginosa virulence. Importantly, phage-injected mice and negative control (non-infected and PBS-injected) mice exhibited 100% survival. On the other hand, all mice infected with P. aeruginosa died at 24 h post inoculation. However, the mortality rate of mice infected with P. aeruginosa and treated with vB_PaeP_PS28 dramatically decreased as compared to mice infected with bacteria alone (Fig. 9a). In addition, bacterial loads were determined in organs isolated from infected mice. As shown in (Fig. 9b, c), the number of viable bacteria in the liver and spleen isolated from mice infected with P. aeruginosa and treated with vB_PaeP_PS28 (3193 ± 12, 5134 ± 13 CFUs/g, respectively) was significantly lower than that of mice infected with P. aeruginosa alone (36 × 104 ± 23, 88 × 104 ± 15 CFUs/g, respectively). Of note that, the phage titers were determined in isolated organs from phage-injected mice as well as mice infected with P. aeruginosa and treated with phage. The phage titer in liver and spleen isolated from P. aeruginosa infected mice and treated with vB_PaeP_PS28 was significantly higher (10,241 ± 23, 8520 ± 13, PFU/mL, respectively) as compared to phage injected mice (1250 ± 11, 1005 ± 10, PFU/mL, respectively). Importantly, the phage vB_PaeP_PS28 was rapidly cleared from the liver and spleen isolated from phage-injected mice. Importantly, the phage-injected mice did not develop any abnormal symptoms over the experiment course. These findings clearly demonstrate that vB_PaeP_PS28 phage is promising for therapeutic use and would be cleared from the body without any harmful effects on the patient.
In vivo characterization of the influence of phage vB_PaeP_PS28 on P. aeruginosa pathogenesis in mice infection model. a Survival curves of mice infected with P. aeruginosa and treated with isolated phage. Mice in first group were infected with P. aeruginosa (2.5 × 107 CFU/mL), mice in second group were injected with vB_PaeP_PS28 (2.5 × 109 PFU/mL) and mice in third group were infected with P. aeruginosa and treated with the phage vB_PaeP_PS28. Uninfected and PBS-injected mice served as negative controls. Mice survival was monitored in each group daily for 4 days and plotted using Kaplan–Meier survival curve. Bacterial burden and phage titers were determined in liver (b) and spleen (c) of infected mice. Mice were anesthetized, liver and spleen were obtained and homogenized for enumeration of CFU and PFU at 24, 48, 72 h post infection. Of note that, bacterial load in P. aeruginosa infected mice was determined at 24 h post infection only as all mice in this group died after 24 h. Bacterial and phage load were represented on left and right y axis, respectively. Data are expressed as means ± SE of three independent experiments
Discussion
P. aeruginosa is responsible for a majority of serious infections including urinary tract and lung infections and pneumonia (Litwin et al. 2021). Due to extensive use of antibiotics and continuous increase of antibiotic resistance, bacteriophages are seemed to be efficient alternatives in management of infections caused by P. aeruginosa (Pires et al. 2015). In the current study, a total of 50 P. aeruginosa isolates were obtained and screened for their antibiotic susceptibility against different antibiotics. P. aeruginosa isolates exhibited a higher resistance towards multiple antibiotics including aminoglycosides, fluoroquinolones and monobactam; revealing that 56% of isolated P. aeruginosa strains were MDR. Therefore, phage therapy could be a promising alternative strategy to control the alarming increase in bacterial resistance of P. aeruginosa. Bacteriophages are supposed to have several advantages compared to antibiotics as being safer and best tolerated without affecting mammalian cells (Kakasis and Panitsa 2019). Furthermore, there is no need for repeated administration of phage doses which commonly known for antibiotics. There is a significant increase in phage concentration at infection site via auto “dosing” that results in greater bacterial killing following only a single dose (Abedon and Thomas-Abedon 2010).
In the current study, a novel lytic phage vB_PaeP_PS28 infecting P. aeruginosa was isolated and characterized. Lytic phages are generally preferred for therapeutic purposes in comparison with temperate phages. Temperate phages exhibit drawbacks including the transfer of virulence genes that could lead to increased antibiotic resistance within bacteria (Monteiro et al. 2019). TEM analysis revealed that vB_PaeP_PS28 is a member of Podoviridae family with icosahedral head and short non contractile tail. Tailed phages with double stranded DNA are classified into three different morphological families; Siphoviridae, Podoviridae and Myoviridae within the order Caudovirales. P. aeruginosa phages have covered all families, and importantly, phages belonging to Podoviridae and Myoviridae families were found to be of major importance and are highly preferred for phage therapy (Alemayehu et al. 2012; Garbe et al. 2011).
The results of host range and EOP analysis are very important parameters that should be determined when selecting bacteriophages for therapeutic purposes (Viscardi et al. 2008). The phage vB_PaeP_PS28 exhibits a broad host range and was able to lyse 13 out of 18 (72.2%) P. aeruginosa strains tested herein. Most of these susceptible strains were highly resistant to traditionally used antibiotics to control P. aeruginosa infections including β-lactams (penicillins, carbapenems, cephalosporins, monobactams), aminoglycosides and fluroquinolones. These findings support the effectiveness of isolated phage and its suitability for application in phage therapy. It is well-known that broad host range phages are considered as efficient biocontrol and more preferable for therapeutic application in phage therapy (Fernández et al. 2019). Moreover, the phage vB_PaeP_PS28 fulfills the requirements of pH and temperature stability and showed a higher stability over a wide temperature and pH ranges. Assessment of phages stability under various temperature and pH conditions is critical to provide information regarding phage storage and application. Phages intended for therapy should be stable under drastic conditions to overcome environmental changes during therapeutic applications (Jamal et al. 2017; Fernández et al. 2019). In agreement with the present findings, tailed phages have been shown to be more stable under harsh conditions including temperature and pH changes (Ackermann et al. 2004). These finding are similar to previous studies reporting the isolation of highly stable phages infecting P. aeruginosa (Danis-Wlodarczyk et al. 2015). In addition to its stability against various conditions, vB_PaeP_PS28 phage possesses a latent period of 15 min and burst size of 210 virions per infected bacterium. These growth characteristics further support the potential incorporation of phage vB_PaeP_PS28 in treatment of P. aeruginosa infections. Phages that have large burst size and short latent period have been reported to be efficient antimicrobial agents (Khan Mirzaei and Nilsson 2015).
Biofilms play an important role in bacterial pathogenesis and could lead to persistence of infections and increased resistance to antibiotics (Mah et al. 2003). Previous reports have demonstrated that bacteriophages are promising in eradicating P. aeruginosa biofilms (Tian et al. 2021). Current results indicate a potent antibiofilm activity of vB_PaeP_PS28 against P. aeruginosa biofilms confirming its suitability for treatment of Pseudomonas infections. The antibiofilm activity of bacteriophages could be related to the production of phage enzymes that degrade polymers in extracellular matrix such as polysaccharides and proteins (Harper et al. 2014). For instance, bacteriophages can encode polysaccharide depolymerase that specifically degrade macromolecular carbohydrates on the host bacterial envelope (Yan et al. 2014). Similarly, bacteriophages produce endolysins which hydrolyse bacterial peptidoglycan, hence inhibit cell wall synthesis (Schmelcher et al. 2012). In addition, bacteriophages could produce enzymes that inhibit quorum sensing in P. aeruginosa and therefore suppress biofilm formation (Whiteley et al. 2018). Interestingly, lytic phages have been found to maintain their lytic activity against persister cells within biofilms which are characterized by low metabolic activity (Tian et al. 2021). Therefore, bacteriophages seem to be a suitable option for the fight against persistent biofilms (Fernández-Barat et al. 2012).
Importantly, the genome of vB_PaeP_PS28 phage was sequenced and gene annotation confirmed that phage vB_PaeP_PS28 is a member of Podoviridae family and the genus Litunavirus of subfamily Migulavirinae. The Litunavirus is a member of a well-characterized N4-like phage. Almost all of the N4-like phages exhibit highly conserved gene organization and expression (Menon et al. 2021; Wittmann et al 2020). The vB_PaeP_PS28 genome encodes gene (ORF 1) which is highly similar to virion-associated RNA polymerase, a remarkable protein that is characteristic of N4-like viruses and unique among all other phages. N4-like viruses co-inject this enzyme with DNA during infection and is responsible for the transcription of early genes (Farmer et al. 2013). The genomes of the N4-like phages from the Pseudomonas group did not reveal any tRNA genes which is in accordance with the annotated vB_PaeP_PS28 phage genome (Wittmann et al 2020). Other genes within phage genome with functional annotation (ORF 8 and ORF 9) have a high percent identity to previously annotated N4-like proteins also supporting the designation of vB_PaeP_PS28 as an N4-like phage. Comparative analysis was performed between vB_PaeP_PS28 phage and other phages infecting P. aeruginosa available in the NCBI. The genomic comparison shows that isolated phage has best similarities with previously isolated and characterized phages; Pseudomonas phage vB_PaeP_FBPa1 (GenBank Acc. No. ON857943.1), Pseudomonas phage vB_PaeS_VL1 (GenBank Acc. No. OK665488.1) and Pseudomonas phage YH6 (GenBank Acc. No. KM974184.1) with percent identity of 94.81, 94.37 and 94.07%; respectively. The results were in accordance with the results of VIRIDIC. These findings were further confirmed upon performing a phylogenetic analysis based on the genes encoding for the terminase large subunit and DNA polymerase that are conserved in different classes of bacteriophages (Shapiro and Putonti 2018; Akhwale et al. 2019). These proteins are considered as helpful phylogenetic markers and routinely used in the investigation of several phage groups and describing the phylogenetic positioning of newly isolated phage (Casjens et al. 2005; Wittmann et al. 2014).
Importantly, no tRNA was found in the vB_PaeP_PS28 genome; this suggests that upon entry into the host, the phage vB_PaeP_PS28 is completely dependent on the host tRNA for its translation machinery. A critical aspect related to bacteriophage therapy is the possibility of transduction where bacteriophages could transfer bacterial virulence genes among bacteria leading to increased bacterial resistance (Mahichi et al. 2009; Sillankorva et al. 2010). Importantly, the genome annotation of phage vB_PaeP_PS28 showed the absence of genes related to lysogenic cycle. These findings confirm that phage vB_PaeP_PS28 is a virulent phage and further support the suitability of this phage for therapeutic applications to combat P. aeruginosa infections.
In the current study, the efficiency of vB_PaeP_PS28 phage to reduce Pseudomonas pathogenesis was assessed in vivo using mice infection model. Intriguingly, there was a significant reduction in the mortality of mice infected with P. aeruginosa and treated with the phage vB_PaeP_PS28 as compared to bacteria-inoculated mice without phage treatment. Moreover, phage treatment effectively reduced bacterial colonization in the organs isolated from Pseudomonas-infected mice relative to mice injected with bacteria alone. Current results are in accordance with previous in vivo studies reporting similar survival rates in Pseudomonas-infected mice following the administration of lytic phages (McVay et al. 2007; Watanabe et al. 2007). Additionally, the phage KPP12 successfully treated Pseudomonas-induced keratitis and markedly reduced bacterial load in infected mice (Fukuda et al. 2012). Rezk et al. (2022) reported that topical application of phage ZCPA1 resulted in complete bacterial eradication in skin wounds and led to efficient wound closure (Rezk et al. 2022). These findings clearly suggest that phage vB_PaeP_PS28 could be a promising antibacterial agent against P. aeruginosa infections.
In conclusion, a lytic phage vB_PaeP_PS28 was isolated belonging to the family Podoviridae that targets P. aeruginosa. The phage vB_PaeP_PS28 exhibits a broad lytic activity as well as higher stability under various environmental conditions. The phage vB_PaeP_PS28 showed a pronounced inhibitory activity against P. aeruginosa planktonic cells as well as a potential antibiofilm activity. The therapeutic efficacy of vB_PaeP_PS28 was investigated in vivo using mice infection model. Treatment with phage vB_PaeP_PS28 markedly reduced mortality in P. aeruginosa-infected mice and lowered bacterial colonization in isolated organs. Intriguingly, based on the genome analysis and in vivo findings, the phage vB_PaeP_PS28 could be a novel promising candidate that can be used in controlling of P. aeruginosa infections. The phage vB_PaeP_PS28 could be introduced as a biocontrol against P. aeruginosa alone or incorporated in phage cocktail therapy. Additionally, the isolated phage could be applied to synergize the action of traditionally used antibiotics targeting P. aeruginosa infections.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- MH:
-
Muller Hinton
- ORFs:
-
Open reading frames
- R:
-
Resistant
- I:
-
Intermediate
- S:
-
Susceptible
- TS:
-
Tryptone soya
- OD:
-
Optical density
- ODc:
-
Cut-off optical density
- TEM:
-
Transmission electron microscopy
- PFUs:
-
Plaque forming units
- CFUs:
-
Colony forming units
- MOI:
-
Multiplicity of infection
- EOP:
-
Efficiency of plating
- MDR:
-
Multidrug resistant
- IP:
-
Intraperitoneally
- ICTV:
-
International committee taxonomy of viruses
- IP:
-
Intraperitoneally
- ICTV:
-
International committee taxonomy of viruses
- BLAST:
-
Basic local alignment search tool
References
Abedon ST, Thomas-Abedon C (2010) Phage therapy pharmacology. Curr Pharm Biotechnol 11(1):28–47. https://doi.org/10.2174/138920110790725410
Abedon ST, García P, Mullany P, Aminov R (2017) Phage therapy: past, present and future. Frontiers Media SA 8:981
Ackermann HW, Tremblay D, Moineau S (2004) Long-term bacteriophage preservation. WFCC Newsl 38:35–40
Adnan M, Shah MRA, Jamal M, Jalil F, Andleeb S, Nawaz MA, Pervez S, Hussain T, Shah I, Imran M (2020) Isolation and characterization of bacteriophage to control multidrug-resistant Pseudomonas aeruginosa planktonic cells and biofilm. Biologicals 63:89–96. https://doi.org/10.1016/j.biologicals.2019.10.003
Adriaenssens EM, Brister JR (2017) How to name and classify your phage: an informal guide. Viruses 9(4):70. https://doi.org/10.3390/v9040070
Akhwale JK, Rohde M, Rohde C, Bunk B, Spröer C, Boga HI, Klenk HP, Wittmann J (2019) Isolation, characterization and analysis of bacteriophages from the haloalkaline lake elmenteita Kenya. PLoS ONE 14(4):e0215734. https://doi.org/10.1371/journal.pone.0215734
Alemayehu D, Casey P, McAuliffe O, Guinane C, Martin J, Shanahan F, Coffey A, Ross R, Hill C (2012) Bacteriophages phiMR299–2 and phiNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. Mbio 3(2):e00029-e112. https://doi.org/10.1128/mBio.00029-12
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Alvi IA, Asif M, Tabassum R, Aslam R, Abbas Z (2020) RLP, a bacteriophage of the family Podoviridae, rescues mice from bacteremia caused by multi-drug-resistant Pseudomonas aeruginosa. Arch Virol 165(6):1289–1297. https://doi.org/10.1007/s00705-020-04601-x
Alvi IA, Asif M, Rehman S (2021) A single dose of a virulent bacteriophage vB PaeP-SaPL, rescues bacteremic mice infected with multi drug resistant Pseudomonas aeruginosa. Virus Res 292:198250. https://doi.org/10.1016/j.virusres.2020.198250
Asif M, Alvi IA, Tabassum R, Rehman SU (2020) TAC1, an unclassified bacteriophage of the family Myoviridae infecting Acinetobacter baumannii with a large burst size and a short latent period. Arch Virol 165(2):419–424. https://doi.org/10.1007/s00705-019-04483-8
Azizian R, Nasser A, Askari H, Kalani MT, Sadeghifard N, Pakzad I, Amini R, Nejad ASM, Jalilian FA (2015) Sewage as a rich source of phage study against Pseudomonas aeruginosa PAO. Biologicals 43(4):238–241. https://doi.org/10.1016/j.biologicals.2015.05.004
Barazandeh M, Shahin K, Hedayatkhah A, Komijani M, Mansoorianfar M. Characterization of a novel bullet-shaped lytic bacteriophage against extensively drug-resistant Pseudomonas aeruginosa isolated from human and domestic sources. Veterinary Research Forum Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. 2021. https://doi.org/10.30466/vrf.2020.110372.2618
Breidenstein EB, de la Fuente-Núñez C, Hancock RE (2011) Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 19(8):419–426. https://doi.org/10.1016/j.tim.2011.04.005
Brown J, Pirrung M, McCue LA (2017) FQC Dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 33(19):3137–3139. https://doi.org/10.1093/bioinformatics/btx373
Cao Z, Zhang J, Niu YD, Cui N, Ma Y, Cao F, Jin L, Li Z, Xu Y (2015) Isolation and characterization of a “phiKMV-like” bacteriophage and its therapeutic effect on mink hemorrhagic pneumonia. PLoS ONE 10(1):e0116571. https://doi.org/10.1371/journal.pone.0116571
Casjens SR, Gilcrease EB, Winn-Stapley DA, Schicklmaier P, Schmieger H, Pedulla ML, Ford ME, Houtz JM, Hatfull GF, Hendrix RW (2005) The generalized transducing Salmonella bacteriophage ES18: complete genome sequence and DNA packaging strategy. J Bacteriol 187(3):1091–1104. https://doi.org/10.1128/JB.187.3.1091-1104.2005
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6(1):17–27. https://doi.org/10.1038/nrmicro1818
Chan PP, Lowe TM (2019) tRNAscan-SE: searching for tRNA genes in genomic sequences. Springer, Gene prediction, pp 1–14
Chen F, Cheng X, Li J, Yuan X, Huang X, Lian M, Li W, Huang T, Xie Y, Liu J (2021) Novel lytic phages protect cells and mice against pseudomonas aeruginosa infection. J Virol 95(8):e01832-e11820. https://doi.org/10.1128/JVI.01832-20
Chen L, Yuan S, Liu Q, Mai G, Yang J, Deng D, Zhang B, Liu C, Ma Y (2018) In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front Microbiol 9:1476. https://doi.org/10.3389/fmicb.2018.01476
Danis-Wlodarczyk K, Olszak T, Arabski M, Wasik S, Majkowska-Skrobek G, Augustyniak D, Gula G, Briers Y, Jang HB, Vandenheuvel D (2015) Characterization of the newly isolated lytic bacteriophages KTN6 and KT28 and their efficacy against Pseudomonas aeruginosa biofilm. PLoS ONE 10(5):e0127603. https://doi.org/10.1371/journal.pone.0127603
de Melo ACC, da Mata GA, Melo FL, Ardisson-Araújo DM, de Vargas APC, Ely VL, Kitajima EW, Ribeiro BM, Wolff JLC (2019) Characterization of a bacteriophage with broad host range against strains of Pseudomonas aeruginosa isolated from domestic animals. BMC Microbiol 19(1):1–15. https://doi.org/10.1186/s12866-019-1481-z
Didamony GE, Askora A, Shehata AA (2015) Isolation and characterization of T7-like lytic bacteriophages infecting multidrug resistant Pseudomonas aeruginosa isolated from Egypt. Curr Microbiol 70(6):786–791. https://doi.org/10.1007/s00284-015-0788-8
Douraghi M, Ghasemi F, Dallal MS, Rahbar M, Rahimiforoushani A (2014) Molecular identification of Pseudomonas aeruginosa recovered from cystic fibrosis patients. J Prev Med Hyg 55(2):50
Dufour N, Delattre R, Ricard JD, Debarbieux L (2017) The lysis of pathogenic Escherichia coli by bacteriophages releases less endotoxin than by β-lactams. Clin Infect Dis 64(11):1582–1588. https://doi.org/10.1093/cid/cix184
English BK, Gaur AH (2010) The use and abuse of antibiotics and the development of antibiotic resistance. Hot topics in infection and immunity in children VI: 73–82.
Enwuru NV, Gill JJ, Anttonen KP, Enwuru CA, Young R, Coker AO, Cirillo JD (2021) Isolation and characterization of novel phage (Podoviridae ɸParuNE1) and its efficacy against multi-drug-resistant Pseudomonas aeruginosa planktonic cells and biofilm. Beni-Suef Univ J Basic Appl Sci 10(1):1–11. https://doi.org/10.1186/s43088-021-00137-4
Fajardo A, Martinez-Martin N, Mercadillo M, Galán JC, Ghysels B, Matthijs S, Cornelis P, Wiehlmann L, Tümmler B, Baquero F (2008) The neglected intrinsic resistome of bacterial pathogens. PLoS ONE 3(2):e1619. https://doi.org/10.1371/journal.pone.0001619
Farmer NG, Wood TL, Chamakura KR, Kuty Everett GF (2013) Complete genome of Acinetobacter baumannii N4-Like Podophage Presley. Genome Announc 1(6):e00852-e1813. https://doi.org/10.1128/genomeA.00852-13
Fernández-Barat L, Ferrer M, Sierra JM, Soy D, Guerrero L, Vila J, Bassi GL, Cortadellas N, Martínez-Olondris P, Rigol M (2012) Linezolid limits burden of methicillin-resistant Staphylococcus aureus in biofilm of tracheal tubes. Crit Care Med 40(8):2385–2389. https://doi.org/10.1097/CCM.0b013e31825332fc
Fernández L, Gutiérrez D, García P, Rodríguez A (2019) The perfect bacteriophage for therapeutic applications—a quick guide. Antibiotics 8(3):126. https://doi.org/10.3390/antibiotics8030126
Fukuda K, Ishida W, Uchiyama J, Rashel M, Kato SI, Morita T, Muraoka A, Sumi T, Matsuzaki S, Daibata M (2012) Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration. PLoS ONE 7(10):e47742. https://doi.org/10.1371/journal.pone.0047742
Gale MJ, Maritato MS, Chen YL, Abdulateef SS, Ruiz JE (2015) Pseudomonas aeruginosa causing inflammatory mass of the nasopharynx in an immunocompromised HIV infected patient: a mimic of malignancy. Idcases 2(2):40–43. https://doi.org/10.1016/j.idcr.2015.01.004
Garbe J, Bunk B, Rohde M, Schobert M (2011) Sequencing and characterization of Pseudomonas aeruginosa phage JG004. BMC Microbiol 11(1):1–12. https://doi.org/10.1186/1471-2180-11-102
Gellatly SL, Hancock RE (2013) Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67(3):159–173. https://doi.org/10.1111/2049-632X.12033
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361(9356):512–519. https://doi.org/10.1016/S0140-6736(03)12489-0
Gurevich A, Saveliev V (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. https://doi.org/10.1093/bioinformatics/btt086
Harper DR, Parracho HM, WalkerJ SR, Hughes G, Werthén M, Lehman S, Morales S (2014) Bacteriophages and biofilms. Antibiotics 3(3):270–284. https://doi.org/10.3390/antibiotics3030270
Humphries RM, Ambler J, Mitchell SL, Castanheira M, Dingle T, Hindler JA, Koeth L, Sei K (2018) CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol 56(4):e01934-e11917. https://doi.org/10.1128/JCM.01934-17
Jamal M, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Das CR (2017) Isolation and characterization of a bacteriophage and its utilization against multi-drug resistant Pseudomonas aeruginosa-2995. Life Sci 190:21–28. https://doi.org/10.1016/j.lfs.2017.09.034
Kakasis A, Panitsa G (2019) Bacteriophage therapy as an alternative treatment for human infections a comprehensive review. Int J Antimicrob Agents 53(1):16–21. https://doi.org/10.1016/j.ijantimicag.2018.09.004
Khan Mirzaei M, Nilsson AS (2015) Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 10(3):e0118557. https://doi.org/10.1371/journal.pone.0118557
Kropinski A, Prangishvili D, Lavigne R (2009) The creation of a rational scheme for the nomenclature of viruses of Bacteria and Archaea. Environ Microbiol. https://doi.org/10.1111/j.1462-2920.2009.01970.x
Krylov V, Shaburova O, Pleteneva E, Krylov S, Kaplan A, Burkaltseva M, Polygach O, Chesnokova E (2015) Selection of phages and conditions for the safe phage therapy against Pseudomonas aeruginosa infections. Virol Sin 30(1):33–44. https://doi.org/10.1007/s12250-014-3546-3
Kumari S, Harjai K, Chhibber S (2009) Characterization of Pseudomonas aeruginosa PAO specific bacteriophages isolated from sewage samples. Am J Biomed Sci 1(2):91–102. https://doi.org/10.5099/aj090200091
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6(1):26–41. https://doi.org/10.1007/s13238-014-0100-x
Litwin A, Rojek S, Gozdzik W, Duszynska W (2021) Pseudomonas aeruginosa device associated–healthcare associated infections and its multidrug resistance at intensive care unit of University Hospital: polish, 8.5-year, prospective, single-centre study. BMC Infect Dis 21(1):1–8. https://doi.org/10.1186/s12879-021-05883-5
Liu J, Gao S, Dong Y, Lu C, Liu Y (2020) Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol 20(1):1–13. https://doi.org/10.1186/s12866-020-01811-w
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426(6964):306–310. https://doi.org/10.1038/nature02122
Mahichi F, Synnott AG, Yamamichi K, Osada T, Tanji Y (2009) Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol Lett 295(2):211–217. https://doi.org/10.1111/j.1574-6968.2009.01588.x
Mazzocco A, Waddell TE, Lingohr E, Johnson RP (2009) Enumeration of bacteriophages by the direct plating plaque assay. Springer, Bacteriophages, pp 77–80
McVay CS, Velásquez M, Fralick JA (2007) Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51(6):1934–1938. https://doi.org/10.1128/AAC.01028-06
Menon ND, Kumar MS, Satheesh Babu T, Bose S, Vijayakumar G, Baswe M, Chatterjee M, D’Silva JR, Shetty K, Haripriyan J (2021) A Novel N4-Like Bacteriophage isolated from a wastewater source in South India with activity against several multidrug-resistant clinical Pseudomonas aeruginosa isolates. Msphere 6(1):e01215-01220. https://doi.org/10.1128/mSphere.01215-20
Miyata R, Yamaguchi K, Uchiyama J, Shigehisa R, Takemura-Uchiyama I, Kato SI, Ujihara T, Sakaguchi Y, Daibata M, Matsuzaki S (2014) Characterization of a novel Pseudomonas aeruginosa bacteriophage, KPP25, of the family Podoviridae. Virus Res 189:43–46. https://doi.org/10.1016/j.virusres.2014.04.019
Monteiro R, Pires DP, Costa AR, Azeredo J (2019) Phage therapy: going temperate? Trends Microbiol 27(4):368–378. https://doi.org/10.1016/j.tim.2018.10.008
Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. https://doi.org/10.3389/fcimb.2017.00039
Moraru C, Varsani A, Kropinski AM (2020) VIRIDIC: a novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses 12(11):1268. https://doi.org/10.3390/v12111268
Morozova V, Kozlova Y, Jdeed G, Tikunov A, Ushakova T, Bardasheva A, Zhirakovskaia E, Poletaeva Y, Ryabchikova E, Tikunova N (2022) A Novel Aeromonas popoffii Phage AerP_220 Proposed to Be a Member of a New Tolavirus Genus in the Autographiviridae Family. Viruses 14(12):2733. https://doi.org/10.3390/v14122733
Namonyo S, Carvalho G, Guo J, Weynberg KD (2022) Novel Bacteriophages Show Activity against Selected Australian Clinical Strains of Pseudomonas aeruginosa. Microorganisms 10(2):210. https://doi.org/10.3390/microorganisms10020210
Patel J, Cockerill F, Bradford P (2015) Performance Standards for Antimicrobial Susceptibility Testing. Clin Lab Stand Inst 35:29
Pires DP, Vilas Boas D, Sillankorva S, Azeredo J (2015) Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol 89(15):7449–7456. https://doi.org/10.1128/JVI.00385-15
Pohane AA, Joshi H, Jain V (2014) Molecular dissection of phage endolysin: an interdomain interaction confers host specificity in Lysin A of Mycobacterium phage D29. J Biol Chem 289(17):12085–12095. https://doi.org/10.1074/jbc.M113.529594
Remold SK, Brown CK, Farris JE, Hundley TC, Perpich JA, Purdy ME (2011) Differential habitat use and niche partitioning by Pseudomonas species in human homes. Microb Ecol 62(3):505–517. https://doi.org/10.1007/s00248-011-9844-5
Rezk N, Abdelsattar AS, Elzoghby D, Agwa MM, Abdelmoteleb M, Aly RG, Fayez MS, Essam K, Zaki BM, El-Shibiny A (2022) Bacteriophage as a potential therapy to control antibiotic-resistant Pseudomonas aeruginosa infection through topical application onto a full-thickness wound in a rat model. J Genet Eng Biotechnol 20(1):1–16. https://doi.org/10.1186/s43141-022-00409-1
Russell DW, Sambrook J (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Cold Spring Harbor, New York
Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7(10):1147–1171. https://doi.org/10.2217/fmb.12.97
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–2069. https://doi.org/10.1093/bioinformatics/btu153
Shapiro JW, Putonti C (2018) Gene co-occurrence networks reflect bacteriophage ecology and evolution. Mbio 9(2):e01870-e11817. https://doi.org/10.1128/mBio.01870-17
Shen M, Le S, Jin X, Li G, Tan Y, Li M, Zhao X, Shen W, Yang Y, Wang J (2016) Characterization and comparative genomic analyses of Pseudomonas aeruginosa phage PaoP5: new members assigned to PAK_P1-like viruses. Sci Rep 6(1):1–6. https://doi.org/10.1038/srep34067
Shigehisa R, Uchiyama J, Kato SI, Takemura-Uchiyama I, Yamaguchi K, Miyata R, Ujihara T, akaguchi Y, Okamoto N, Shimakura H (2016) Characterization of Pseudomonas aeruginosa phage KPP21 belonging to family Podoviridae genus N4-like viruses isolated in Japan. Microbiol Immunol 60(1):64–67. https://doi.org/10.1111/1348-0421.12347
Sillankorva S, Neubauer P, Azeredo J (2010) Phage control of dual species biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling 26(5):567–575. https://doi.org/10.1080/08927014.2010.494251
Skurnik M, Pajunen M, Kiljunen S (2007) Biotechnological challenges of phage therapy. Biotechnol Lett 29(7):995–1003. https://doi.org/10.1007/s10529-007-9346-1
Stepanović S, Vuković D, Hola V, Bonaventura GD, Djukić S, Ćirković I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x
Stothard P, Wishart DS (2005) Circular genome visualization and exploration using CGView. Bioinformatics 21(4):537–539. https://doi.org/10.1093/bioinformatics/bti054
Sullivan MJ, Petty NK, Beatson SA (2011) Easyfig: a genome comparison visualizer. Bioinformatics 27(7):1009–1010. https://doi.org/10.1093/bioinformatics/btr039
Taati Moghadam M, Khoshbayan A, Chegini Z, Farahani I, Shariati A (2020) Bacteriophages, a new therapeutic solution for inhibiting multidrug-resistant bacteria causing wound infection: lesson from animal models and clinical trials. Drug Des Devel Ther. https://doi.org/10.2147/DDDT.S251171
Tang C, Deng C, Zhang Y, Xiao C, Wang J, Rao X, Hu F, Lu S (2018) Characterization and genomic analyses of Pseudomonas aeruginosa Podovirus TC6: establishment of genus Pa11virus. Front Microbiol 9:2561. https://doi.org/10.3389/fmicb.2018.02561
Thanh NC, Nagayoshi Y, Fujino Y, Iiyama K, Furuya N, Hiromasa Y, Iwamoto T, Doi K (2020) Characterization and genome structure of virulent phage EspM4VN to control Enterobacter sp M4 isolated from plant soft rot. Front Microbiol 11:885. https://doi.org/10.3389/fmicb.2020.00885
Tian F, Li J, Nazir A, Tong Y (2021) Bacteriophage–a promising alternative measure for bacterial biofilm control. Infect Drug Resist 14:205. https://doi.org/10.2147/IDR.S290093
Viscardi M, Perugini AG, Auriemma C, Capuano F, Morabito S, Kim KP, Loessner MJ, Iovane G (2008) Isolation and characterisation of two novel coliphages with high potential to control antibiotic-resistant pathogenic Escherichia coli (EHEC and EPEC). Int J Antimicrob Agents 31(2):152–157. https://doi.org/10.1016/j.ijantimicag.2007.09.007
Watanabe R, Matsumoto T, Sano G, Ishii Y, Tateda K, Sumiyama Y, Uchiyama J, Sakurai S, Matsuzaki S, Imai S (2007) Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother 51(2):446–452. https://doi.org/10.1128/AAC.00635-06
Whiteley M, Diggle SP, Greenberg EP (2018) Correction: corrigendum: progress in and promise of bacterial quorum sensing research. Nature 555(7694):126–126. https://doi.org/10.1038/nature24624
Wittmann J, Turner D, Millard AD, Mahadevan P, Adriaenssens KAM (2020) From orphan phage to a proposed new family–the diversity of N4-like viruses. Antibiotics 9(10):663. https://doi.org/10.3390/antibiotics9100663
Wick RR, Judd LM, Gorrie CL, Holt KE (2017) Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom. https://doi.org/10.1099/mgen.0.000132
Wittmann J, Dreiseikelmann B, Rohde C, Rohde M, Sikorski J (2014) Isolation and characterization of numerous novel phages targeting diverse strains of the ubiquitous and opportunistic pathogen Achromobacter xylosoxidans. PLoS ONE 9(1):e86935. https://doi.org/10.1371/journal.pone.0086935
Yan J, Mao J, Xie J (2014) Bacteriophage polysaccharide depolymerases and biomedical applications. BioDrugs 28(3):265–274. https://doi.org/10.1007/s40259-013-0081-y
Acknowledgements
The authors would like to acknowledge the medical staff at clinical laboratories of Zagazig University Hospitals in Zagazig, Egypt for providing clinical Pseudomonas aeruginosa isolates for this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, AE, GS, and MA; methodology, AA, and MA; validation, AA, and MA; investigation, AE, GS, and MA; data curation, AA, and MA; writing-original draft preparation, AA, and MA; writing-review and editing, AA, and MA; visualization, AA, AE, GS, and MA; supervision, GS, AE, and MA; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out according to the Declaration of Helsinki, and informed consent has been obtained from the subjects. The Institutional Review Board (IRB) provided the ethical approval under the number (ZU-IRB #10219). All mentioned procedures in the animal study were performed according to Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press) and the ethical standards of the Zagazig University-Institutional Animal Care and Use Committee (ZU-IACUC), with approval number (ZU-IACUC/3/F/72/2022).
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Antibiotic susceptibility of P. aeruginosa isolates. Table S2. Antibiotic sensitivity and phage susceptibility of P. aeruginosa isolates from different clinical sources tested for host range determination of vB_PaeP_PS28. Table S3. Bacterial and phage count following infection of host and P. aeruginosa PAO1 with vB_PaeP_PS28. Fig. S1. Quantitative evaluation of P. aeruginosa biofilm formation. Fig. S2. Dot Plot comparisons of the genomic nucleotide sequences of vB_PaeP_PS28 and related bacteriophages infecting P. aeruginosa.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelghafar, A., El-Ganiny, A., Shaker, G. et al. Isolation of a bacteriophage targeting Pseudomonas aeruginosa and exhibits a promising in vivo efficacy. AMB Expr 13, 79 (2023). https://doi.org/10.1186/s13568-023-01582-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-023-01582-3