- Research
- Open access
- Published:
Increased tuberculosis case detection in Tanzanian children and adults using African giant pouched rats
BMC Infectious Diseases volume 24, Article number: 401 (2024)
Abstract
Background
African giant pouched rats, trained by Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO), have demonstrated their ability to detect tuberculosis (TB) from sputum. We assessed rat-based case detection and compared the mycobacterium bacillary load (MTB-load) in children versus adults.
Methods
From January–December 2022, samples were collected prospectively from 69 Directly Observed Therapy (DOT) facilities’ presumed TB patients. Using an average of five rats, APOPO re-evaluated patients with bacteriologically negative (sputum-smear microscopy or Xpert MTB/RIF) results. Rat-positive samples were tested using concentrated smear light-emitting diode microscopy to confirm TB detection before treatment initiation. The rats’ identification of pulmonary TB is based on smelling TB-specific volatile organic compounds (VOCs) in sputum. Using STATA, Chi-square for odds ratio and confidence interval was calculated and evaluated: (1) the yield of rat-based TB detection compared to that of the health facilities; (2) rat-based TB detection in children versus adults; and (3) rats’ ability to detect TB across MTB-loads and between children and adults.
Results
From 35,766 patients, 5.3% (1900/35,766) were smear-positive and 94.7% (33,866/35,766) were smear or Xpert-negatives at DOTS facility. Of those with negative results, 2029 TB cases were detected using rats, contributing to 52% (2029/3929 of total TB identified), which otherwise would have been missed. Compared to DOT facilities, rats were six-fold more likely to detect TB among Acid Fast Bacilli (AFB) 1+/scanty [90% (1829/2029) versus 60% (1139/1900), odds ratio, OR = 6.11, 95% confidence interval, CI: 5.14–7.26]; twice more likely to identify TB cases among children [71% (91/129) versus 51% (1795/3542), OR = 2.3, 95% CI: 1.59–3.42]; and twice more likely to identify TB cases among children with AFB 1+/scanty than adults with the same MTB-load [5% (86/1703) versus 3% (28/1067), OR = 2.0, 95% CI: 1.28–3.03].
Conclusions
Rats contributed over half of the TB cases identified in program settings, and children, especially those with a lower MTB-load, were more likely to be diagnosed with TB by rats. The chemical signatures, VOCs, were only available for adults, and further research describing the characteristics of VOCs in children versus adults may pave the way to enhance TB diagnosis in children.
Introduction
Despite the curable and preventable nature of tuberculosis (TB), about 10.6 million people fell ill with it and 1.6 million died in 2021 [1]. The African region accounts for nearly one-third of the estimated global burden of TB, and TB remains a public health problem in sub-Saharan African countries, Tanzania included. TB is declining by only 5% on average annually in Tanzania [1]. With this current rate of decline, considerable gaps persist in finding and treating TB cases timely, as a result of which achieving reduced TB transmission and TB cases per the END TB target may not be possible in the near future [2]. Therefore, scaling up of cheaper, faster, and more sustainable diagnostic tests to find more TB patients, especially those missed in routine program settings, is essential.
Over the years, African giant pouched rats (Cricetomys ansorgei), trained by Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO) to identify TB by smell, have demonstrated their ability to detect TB from sputum [3,4,5]. The trained rats have very high sample throughput, as they can screen 100 sputum samples in 20 minutes [6]. The rats’ identification of pulmonary TB is based on smelling TB-specific volatile organic compounds (VOCs) in sputum specimens [5]. By the end of 2022, APOPO’s TB Detection Programs had screened 870,777 sputum samples from 517,264 presumptive TB patients and found 26,084 newly diagnosed TB patients in three high-TB burden countries (Ethiopia, Mozambique, and Tanzania). At APOPO, we have generally done this by re-evaluating (second-line TB screening) sputum samples initially tested and declared bacteriologically negative by sputum-smear microscopy (smear) or Xpert MTB/RIF [4]. Before treatment initiation, all TB patients identified using rats are confirmed using the national standard diagnostics.
Due to the low bacillary load and lesser sensitivity of the available diagnostic methods, including culture, diagnosing TB in children is difficult, leaving them undiagnosed and therefore untreated [7]. Using APOPO rats, Mgode et al. reported on the diagnosis of TB in children (0–14 years) in 2018. They discovered 39% (208/539) contributions among pediatric TB cases reported, which translates to 62.8% additional cases (incremental yield) by rats to DOT facilities TB case detection [8]. There is limited data on rats’ abilities to detect TB in children versus adults. Other than the above report, all the previous studies were focused on adults [9, 10]. In this study, we aim to evaluate: (1) the yield of rat-based TB case detection compared to that of the health facilities; (2) rat-based TB case detection in children versus adults; and (3) rats’ ability to detect TB across Mycobacterium TB bacillary loads (MTB-load) and between children and adults.
Methods
Study settings and designs
We conducted a prospective study using routinely collected data on presumptive TB patients enrolled in the national TB program’s routine care at 69 (58 Dar es Salaam, 11 Dodoma) DOT facilities from 1st of January to 31st of December 2022 in Tanzania.
Study population
The study population includes all individuals of all ages, including children, men, and women, who presented with symptoms of TB at study sites.
Tuberculosis screening
At the health facility visit, children (0–14 years old) and adults (15 years and above, hereafter referred to as adults) were screened for TB symptoms. Per the national TB guidelines, adults were screened for 4 TB symptoms (cough, fever, night sweats, and weight loss) of two or more weeks duration. Children were screened for weight loss or failure to thrive (no weight gain > 3 months), cough for ≥2 weeks, fever for ≥2 weeks, fatigue or reduced playfulness for ≥2 weeks, and profuse night sweats for ≥2 weeks [11]. Presumptive TB cases were defined when patients were screened positive for one or more of the TB symptoms using the above guide.
APOPO tuberculosis detection model using rats
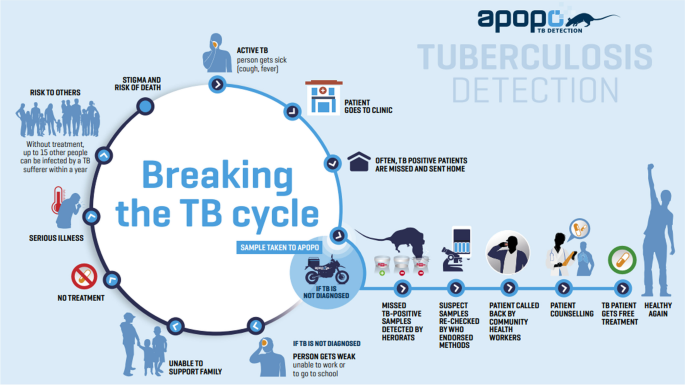
APOPO sample collection, a rat-based assessment procedure, and a TB detection model using rats were reported in previous APOPO publications (Fig. 1) [3, 12]. In summary, smear, which has very good specificity but very poor sensitivity, especially in sub-Saharan African settings [13], remains the most commonly used TB diagnostic method in low- and middle-income countries [4]. At APOPO, samples from smear- or Xpert MTB/RIF-negative presumptive TB patients are screened using detection rats, and rat-positive samples are confirmed using light-emitting diode fluorescence smear microscopy (LED-FM), yielding an annual average of a 40% increase (incremental yield) in smear-positive case detection [3]. Therefore, APOPO envisages targeting and reaching the missed TB cases to maximize TB case detection and treatment coverage. In this study, five rats on average sequentially evaluated the samples placed under 10 sniffing holes in the floor in a of rectangular chamber (205 cm long, 55 cm wide, and 55 cm high). The details of the evaluation setup have been reported elsewhere [14].
APOPO tuberculosis detection model using rats. Reproduced with permission [12]
Data collection
Data were collected using standardized case report forms (CRF) between January and December 2022. An APOPO-developed TB Laboratory Information System (TB-LIMS) was used to record the data. Logic checks were used to find inconsistencies, which were then verified against the original CRF after being discovered. Consistencies and missing data were, whenever possible, fixed by reviewing patient charts.
Statistical analysis
Data were analyzed using STATA statistical software 15 [15]. A Chi-square for odds ratio was calculated to analyze the demographic and laboratory parameters of the patients, and rats’ TB detection over the DOT facilities and compared the increase by MTB-load in children versus adults. We employed an odds ratio and a 95% confidence interval, and statistical significance was determined by P values less than 0.05.
Results
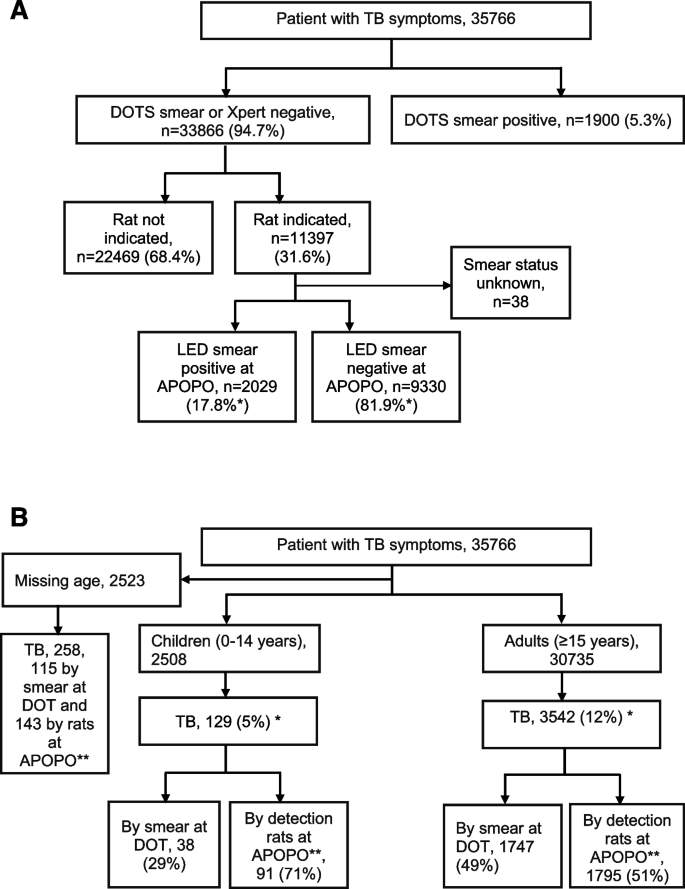
From a total of 35,766 patients, 46,048 samples were screened between January and December 2022; Among these patients 5.3% (1900/35,766) were smear-positive and 94.7% (33,866/35,766) were smear or Xpert-negatives at DOTS facility (Fig. 2a). Figure 2b shows children and adults, respectively, were 8% (2508/33,243) and 92% (30,735/33,243). Among presumptive TB patients 2523, and 258 TB patients were not included for the odds ratio analysis due to missing age. Bacteriologically confirmed (smear at DOT facilities and rats’ detection verified by smear) cumulative TB cases were 11% (3929/35,766), whereas 5% (129/2508) and 12% (3542/30,735) were among children and adults, respectively. The median age was 36 years (interquartile range, 26–47 years), and 68% (2680/3903) were males (Fig. 2b and Table 1).
A Rat indication and tuberculosis cases after LED smear confirmation among presumptive tuberculosis patients in Tanzania study sites from January to December 2022. Note: *Since the samples were not tested by culture, 17.8% does not truly represent sensitivity. Since the LED smear is not good enough in sensitivity, nor does 81.9% represent a true false positive. B Presumptive tuberculosis and bacteriologically confirmed tuberculosis cases in Tanzania study sites from January to December 2022. Note: *Children versus adults bacteriologically confirmed TB: 5% (129/2508) versus 12% (3542/30,735), odds ratio 0.42, 95% confidence interval: 0.35–0.50, p value, 0.001
TB detection using rats
Of the 3929 TB cases, APOPO rats’ detection contributed 52% (2029/3929) of the TB cases reported as smear-positive TB at DOT facilities that otherwise would have been missed by routine program. Compared to DOT facilities, the detection rats were six-fold more likely to detect TB among patients with Acid Fast Bacilli (AFB) smear 1+ or scanty [90% (1829/2029) versus 60% (1139/1900), odds ratio, OR = 6.11, 95% confidence interval, CI: 5.14–7.26]. The odds of identifying TB cases by rat among children were two-fold higher than adults [71% (91/129) versus 51% (1795/3542), OR = 2.3, 95% CI: 1.59–3.42] (Table 1). Furthermore, the odds of identifying TB cases by rats among children with AFB 1+ or scanty smear were two-fold higher than those among adults with the same MTB-load range [5% (86/1703) versus 3% (28/1067), OR = 2.0, 95% CI: 1.28–3.03] (Tables 2 and 3 and Figs. 3 and 4).
Tuberculosis detection by smear at DOTs and by rats at APOPO confirmed by LED smear by Mycobacterium tuberculosis bacillary load
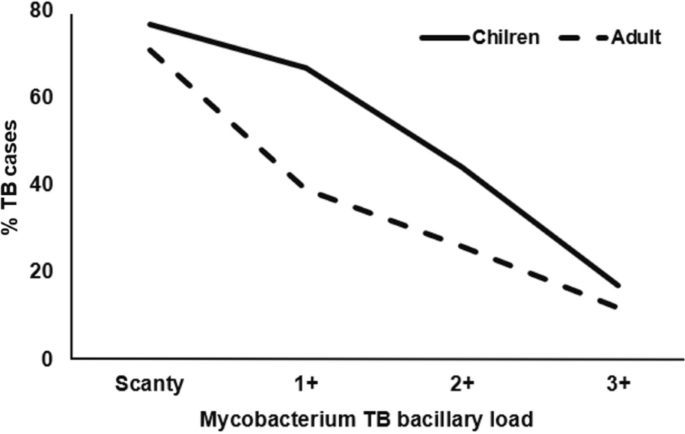
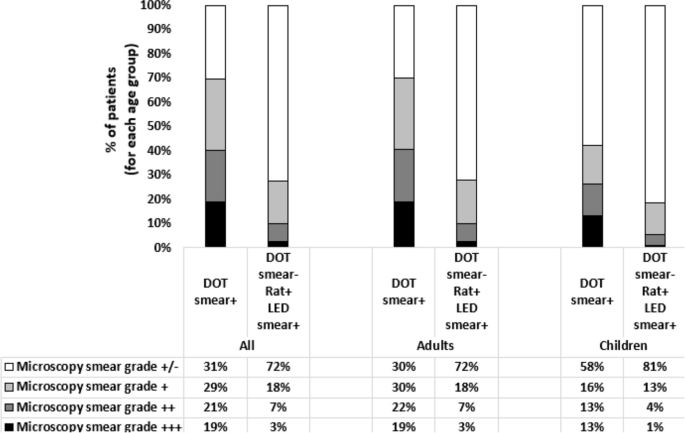
Higher proportions of AFB Scanty were identified among TB diagnosed by rats and confirmed by LED smear than those diagnosed at DOT, and this was similar among children and adults (Fig. 4).
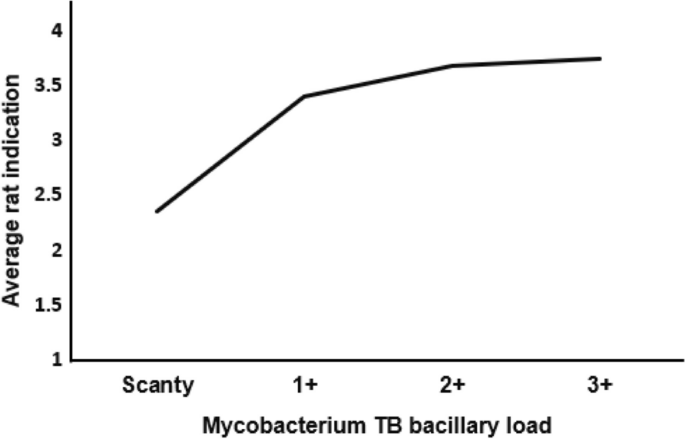
On average, 2.36 (3459/1466), 3.40 (1233/363), 3.68 (545/148), and 3.75 (195/52) rats from a set of five rats indicated TB in those with AFB Scanty, 1+, 2+, and 3+, respectively More rats were able to identify TB as the MTB-load increased (Fig. 5).
Discussions
For more than a decade, APOPO-trained African giant pouched rats have been used to re-evaluate (second-line screening) sputum samples tested by smear under routine program conditions [3, 4], and this has allowed us to compare the effect of rats on pulmonary TB case finding against DOT facilities. In the present study, rats demonstrated a 52% contribution in the detection of TB cases among patients who presented to routine DOT facilities with symptoms of TB and were diagnosed with TB. Rats were six times more likely than DOT facilities to uncover TB in patients with AFB smear 1+ or scanty, and this finding is similar to a previous study (five times more likely), though the latter was only applicable to children [4]. It is worth noting that if verification tests following the sputum samples’ rat indication as positive had been confirmed by molecular WHO-recommended rapid diagnostics, such as Xpert MTB/RIF Untra or Truenat, or by culture, which can detect TB with much lower concentrations of mycobacterium in a sample than concentrated LED microscopy, the contribution of rats would have been higher. Rats’ contribution has implications for Tanzania’s efforts to eliminate TB, especially as those who were identified using rats were deemed TB negative (missed TB cases) in the context of the standard TB diagnostic procedures and would have been left untreated otherwise.
We described elsewhere how rats can discriminate Mycobacterium TB by sniffing a variety of VOCs [3, 5]. According to a recent meta-analysis (53,181 samples from 24,600 patients in seven studies), the sensitivity and specificity of TB screening using rats were 81.3 and 73.4%, respectively [16]. In our report, we further analyzed the rats’ responses to different MTB-loads with respect to their impact on children and adults with pulmonary TB.
TB detection using rats among children and adults
Comparing the effect of TB detection using rats in children to that in adults, rats were more than twice as likely to identify TB in children compared to adults, even though the likelihood of TB diagnosis among children with presumptive TB was lower by 58%. It should be noted that the increased TB detection among children was not only higher, but it was also significantly higher among children with a lesser bacillary load (AFB 1+ or scanty) compared to adults with a similar bacillary load. Our finding suggests that the rat-based technology could be used to improve pediatric TB case detection in high-burden countries such as Tanzania, where children are diagnosed with TB less frequently than adults by the standard of care.
In the previous study, rats contributed 39% (208/539) to pulmonary TB case detection reported in children (0–14 years), which was far less than our finding (71%, p < 0.001) [8].
APOPO’s rat technology has shown further improvement over time toward better rat and rat-handler training techniques, among other possibilities, which may explain the difference between the two reports. APOPO is working to advance its technologies further in this direction, and rats’ performance-rewarding systems are being computerized. The recent fully automated (i.e., controlling indication threshold time, notification of correct identification, reward delivery, and data recording are all computer controlled) rat cage is one step in this process and is currently being evaluated [14]. If validated, the automated system eliminates the effect of the human factor, i.e., rat handlers, who previously rewarded rats manually by providing food after a rat indicated the sample had TB. With the automated cage, APOPO anticipates improving and speeding up TB detection in Tanzania. In agreement with Mgode et al., our work has implications for developing rat-based TB diagnostic techniques or algorithms among children due to the challenges of detecting TB in this age group, where sputum quality and quantity remain a concern [7, 8].
Fewer number of rats is needed to identify TB with a higher bacillary load
The direct relationship between smear positivity and MTB-load was expected [17]. Interestingly, we also observed that when the bacillary load increased, more and more rats identified TB cases, suggesting that fewer rats were needed to detect TB, and once again, the effect tends to be stronger in children than in adults. The properties of the chemical signature, VOCs, among various age groups have not yet been determined, and the previous report was for adults only [5]. Our research led us to two hypotheses: (1) children may have distinct or greater VOC characteristics, making it simpler to diagnose TB even when the bacillary load is lower than that of adults; and (2) probably, the less developed physiology may not let them to produce other odors that could be developed by adult patients, which in turn may somehow mask the odor from the characteristic VOCs. These merit additional investigation. On the other hand, sputum quality and quantity have an impact on the accuracy of TB diagnosis by smear or Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) [17,18,19], and in the same vein, higher-quality sputum makes it simpler for rats to detect TB.
Our study has some limitations. First, because the data were from a routine program setting, the age of certain TB cases was missing, which prevented them from being further analyzed. Rats were, however, four times more likely to detect TB than DOT among those with missing ages, and It’s likely that the conclusion would remain unchanged if the missing data were included. Second, health workers at various DOT facilities may not have received training before the data collection, and the TB screening and recording procedures may not have been consistent, which may result in inconsistent presumptive patient identification. Third, despite the fact that sputum induction is usual at DOT facilities, it was possible to collect insufficient sputum samples from children, which may have resulted in fewer pediatric TB diagnoses than in adult patients. Finally, for those samples evaluated by rats after the Xpert MTB/RIF negative test result, using the same sample for rats was not possible. Thus, a sister sample was collected and evaluated by rats. In such cases, we cannot rule out the possibility of inter-sample variations, i.e., the sister sample had a chance not to be negative if it was tested by Xpert MTB/RIF before rats’ evaluation.
In conclusion, in our high TB burden settings, APOPO rats led to more than half of the TB cases identified. Children had a higher rate of TB detection, particularly among those with lower bacillary loads. In the present study, rats demonstrated their essential role in TB control efforts, specifically in identifying TB cases that were overlooked in routine program diagnostic settings. Since rats were more easily able to detect TB in children than adults, and the data from prior reports about chemical signatures, VOCs, were only available for adults, further research describing the characteristics of VOCs in children versus adults may pave the way to enhance TB diagnosis in children. Like other TB diagnostic technologies, it’s likely that the quality of the sputum specimen influences the rat’s ability to detect TB by sniffing; therefore, advancements in this area make it simpler for the rats to recognize TB.
Availability of data and materials
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Although the patient-level data do not include patient names, this IRB decision is in the interest of ensuring patient confidentiality. An individual may email the lead author (tefersast@gmail.com) or the APOPO TB Detection Research Department (tefera.agizew@apopo.org) to request the data.
Abbreviations
- AFB:
-
Acid fast bacilli
- APOPO:
-
Anti-persoonsmijnen ontmijnende product ontwikkeling
- CI:
-
Confidence interval
- CRF:
-
Case report form
- DOT:
-
Directly observed therapy
- LED-FM:
-
Light-emitting diode fluorescence smear microscopy
- MTB:
-
Mycobacterium Tuberculosis
- OR:
-
Odds ratio
- RIF:
-
Rifampicin
- STATA:
-
South Texas Art Therapy Association
- TB:
-
Tuberculosis
- VOC:
-
Volatile organic compound
- ZN:
-
Ziehl-Neelsen
References
World Health Organization (WHO). Global TB report 2022. Geneva, Switzerland: WHO; 2022. Accessed on 10 July 2023, and available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
Agizew TB, Dememew ZG, Leta T, Hiruy H, Tesema E, Abelti EA, et al. Prospects for tuberculosis elimination in Ethiopia: feasibility, challenges, and opportunities. Pan Afr Med J. 2022;43(146) https://doi.org/10.11604/pamj.2022.43.146.35557.
Mulder C, Mgode GF, Ellis H, Valverde E, Beyene N, Cox C, et al. Accuracy of giant African pouched rats for diagnosing tuberculosis: comparison with culture and Xpert MTB/RIF. Int J Tuberc Lung Dis. 2017;21(11):1127–33. https://doi.org/10.5588/ijtld.17.0139.
Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (APOPO). Annual report 2022. Morogoro, Tanzania; 2022. Accessed on 1 July 2023, and available from: https://apopo.org
Mgode GF, Weetjens BJ, Nawrath T, Lazar D, Cox C, Jubitana M, et al. Mycobacterium tuberculosis volatile s for diagnosis of tuberculosis by Cricetomys rats. Tuberculosis. 2012;92:535–42. https://doi.org/10.1016/j.tube.2012.07.006.
Mulder C, Mgode G, Reid SE. Tuberculosis diagnostic technology: an African solution. . .think rats. Afr J Lab Med. 2017;6:a420. https://doi.org/10.4102/ajlm.v6i2.420.
Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Intl J Tuberc Lung Dis. 2001;5:594–603. https://pubmed.ncbi.nlm.nih.gov/11467365/
Mgode GF, Cox LC, Mwimanzi S, Mulder C. Pediatric tuberculosis detection using trained African giant pouched rats. Pediatr Res. 2018;84:99–103. https://doi.org/10.1038/pr.2018.40.
Poling A, Weetjens BJ, Cox C, Mgode G, Jubitana M, Kazwala R, et al. Using giant African pouched rats to detect tuberculosis in human sputum samples: 2009 findings. Am J Trop Med Hyg. 2010;83(6):1308–10. https://doi.org/10.4269/ajtmh.2010.10-0180.
Mahoney AM, Weetjens BJ, Cox C, et al. Using giant African pouched rats to detect tuberculosis in human sputum samples: 2010 findings. Pan Afr Med J. 2011;9:28. https://doi.org/10.4269/ajtmh.2010.10-0180.
The United Republic of Tanzania, National Tuberculosis and leprosy program., National Tuberculosis Program Manual, Ministry of Health, Tanzania 2020. Accessed on 11 Sept 2023, and available from: https://ntlp.go.tz/site/assets/files/1081/ntlp_manual_2020_2021_1.pdf
Beyene N, Sitotaw LA, Telila EG, Gebre HA, Alemu NB, Burny R, et. al. The use of rats to detect drug-resistant TB. PHA Mach 2023; 13(1):64–69. https://doi.org/10.5588/pha.22.0059.
Alnour TMS. Smear microscopy as a diagnostic tool of tuberculosis: review of smear negative cases, frequency, risk factors, and prevention criteria. Indian J Tuberc. 2018;65(3):190–4. https://doi.org/10.1016/j.ijtb.2018.02.001.
Beyene N, Mgode G, Burny R, Fast CD, Cox C, Fiebig L. A multidisciplinary approach towards finding and treating all tuberculosis patients. In: Rezaei N, editor. Tuberculosis: integrated studies for a complex disease. Switzerland: Springer-Verlag; 2023. p. 207–27.
StataCorp. Stata statistical software: release 14. College Station, TX: StataCorp LP; 2015.
Kanaan R, Farkas N, Heygi P, Soós A, Hegyi D, Németh K, et al. Rats sniff out pulmonary tuberculosis from sputum: a diagnostic accuracy meta-analysis. Sci Rep. 2021;11(1):1877. https://doi.org/10.1038/s41598-021-81086-x.
Lumb R, Van-Deun A, Ivan Bastian I, Mark F-GM. Laboratory diagnosis of tuberculosis by sputum microscopy: the handbook. Australia: global edition. SA Pathology: Adelaide, South Australia; 2013. https://catalogue.nla.gov.au/catalog/6448252
World Health Organization (WHO) same-day diagnosis of tuberculosis by microscopy 2011. WHO/HTM/TB/2011.7. Accessed on 17 Aug 2023, and available from: https://www.who.int/publications/i/item/WHO-HTM-TB-2011.7
Zimba O, Tamuhla T, Basotli J, Letsibogo G, Pals S, Mathebula U, et al. The effect of sputum quality and volume on the yield of bacteriologically-confirmed TB by Xpert MTB/RIF and smear microscopy among people living with HIV in Botswana. The Pan Afri Med Jour. 2019;33:110. https://doi.org/10.11604/pamj.2019.33.110.15319.
Acknowledgements
The authors gratefully acknowledge the service of the APOPO TB research team, which carried out the day-to-day work of the evaluation of samples from DOT facilities. The authors would also like to thank the Tanzania Ministry of Health, the National TB and Leprosy Program, and health care workers at the collaborating DOT facilities in Dar es Salaam and Dodoma. Patient tracking by MKUTA is much appreciated.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the APOPO project. References in this manuscript to any specific commercial products, process, service, manufacturer, or company do not constitute its endorsement or recommendation by the donors or APOPO.
Funding
This research has been supported by Else Kröner-Fresenius-Stiftung (EKFS, Germany), the Light Foundation (Germany), the Polish embassy in Tanzania, and the Directorate General for Development Cooperation (DGD, Belgium), who supported the study financially. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.A and J.S conceptualized and designed the study and conducted the statistical analysis. S.M, G.M, N.E, M.S., and R.K conducted the fieldwork and laboratory work. T.A, J.S, S.M, C.F, R.B, C.C, and N.B reviewed the collected data and participated in the writing of the results and discussions. All co-authors contributed to the writing and review of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board (IRB, Ref. nr. NMRI/HQ/R.8c/Vov.I/2066) at the Tanzanian National Institute for Medical Research (Dar es Salaam, Tanzania). Following IRB approval, patients were enrolled in the study, and data were collected as part of the national TB program’s routine TB diagnostic care activities. Therefore, the ethical committee waived the need for informed consent for this operational research that involved second-line TB screening following routine care.
Pertaining to the rats, all procedures were conducted with approval from the Institutional Committee for Research Involving Animals of the Sokoine University of Agriculture and in accordance with all relevant guidelines and regulations. The methods and results reported herein adhere to the National Centre for Replacement Refinement & Reduction of Animals in Research ARRIVE 2.0 guidelines. At the conclusion of all experiments, subjects remained housed according to the procedures described above with no animals sacrificed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Agizew, T.B., Soka, J., Fast, C.D. et al. Increased tuberculosis case detection in Tanzanian children and adults using African giant pouched rats. BMC Infect Dis 24, 401 (2024). https://doi.org/10.1186/s12879-024-09313-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09313-0