Abstract
Molybdenum phosphide in transition metal phosphides members are considered as an attractive electrocatalyst for hydrogen evolution reaction (HER). However, its unsatisfactory stability and conductivity in an alkaline environment has dragged on its development. Here, we successfully introduced N, C co-doped MoP (MoP-NC) nanoparticles by a simple and efficient two-step synthesis method using urea as a carbon source into the molybdenum phosphide system. The cheapness of urea and the excellent carbon to nitrogen ratio remove the obstacles ahead of the development of MoP-NC composites. The obtained composites have excellent HER electrocatalytic activity and stability in 1-M potassium hydroxide (KOH) solution, which requires only an overpotential of 131 mV to achieve a current density of 10 mA cm−2 and exhibits negligible performance degradation after 1000 CV cycles.
Similar content being viewed by others

Background
In recent years, the rapid development of human beings has led to the gradual depletion of fossil energy [1,2,3]. Therefore, researchers are striving to find an environmentally friendly energy source to curb this vicious circle, so that hydrogen can be held on the throne of energy. However, traditional photolysis and electrolysis of water to produce hydrogen are in a difficult bottleneck in terms of efficiency. Whereas with the introduction of high-efficiency electrolyzed water-catalyzed hydrogen production catalysts, people have made a qualitative leap in the mass production of hydrogen. This catalyst-mediated electrocatalytic process requires the catalyst itself to have low hydrogen evolution reaction (HER) overpotential. Although the noble metals such as Pt at this stage have extremely low overpotential and excellent stability, they are expensive [4,5,6]. The scarcity of available soil limits the large-scale application of such catalysts [7,8,9]. Therefore, finding a material with low cost and relatively good electrocatalytic hydrogen evolution performance has been a hot spot in the past few years [10,11,12,13].
It is worth noting that recently researchers have found that some non-precious metal catalysts have a good price/performance ratio in the direction of hydrogen evolution, among which the most widely used Molybdenum phosphide (MoP) in transition metal phosphides (TMPs) [14,15,16,17]. Sun et al. mixed (NH4)6Mo7O24·4H2O, (NH4)2HPO4 and citric acid (CA) in different molar ratios, when Mo:P:CA = 1:1:x and x = 2, the formation of crosslinked network structure MoP nanoparticles have the best HER performance [18]. Joshua et al. prepared amorphous MoP nanoparticles with good HER properties by heating hexacarbonyl molybdenum and trioctylphosphine (TOP) [19]. However, the conductivity of pure molybdenum phosphide is not satisfactory, and the hydrogen evolution performance and stability in an alkaline solution are not as good as in an acidic environment, so that the conductivity and stability can be enhanced by introducing a carbon-based material [20,21,22].
We achieved high-efficiency two-step synthesis by introducing urea as a carbon source in the molybdenum phosphide system, and successfully prepared N, C co-doped MoP (MoP-NC) nanoparticles, which have excellent catalytic activity and stability even in alkaline electrolytes. In addition, we designed two control groups that explored urea action including no carbon source and glucose instead of urea as the carbon source. Interestingly, the former is always weaker than the latter when using glucose and urea as carbon sources, respectively. This can be attributed to the role of urea as both a carbon source and a nitrogen source for the auxiliary synthesis of molybdenum phosphide [23].
Presentation of the Hypothesis
Molybdenum phosphide is widely used as non-precious metal catalyst in the direction of hydrogen evolution. The introduction of carbon source can improve the conductivity and stability of the electrocatalyst. The introduction of nitrogen source can improve the hydrogen evolution performance in an alkaline solution.
Testing the Hypothesis
Materials
Urea (CH4N2O), glucose (C6H12O6), ammonium dihydrogen phosphate (NH4H2PO4), and ammonium heptamolybdate ((NH4)6Mo7O24·4H2O) were purchased from Sinopharm Chemical Reagent Co., Ltd. KOH was purchased from Aladdin Ltd. in Shanghai. Deionized water used in the experiment was from ultrapure water equipment.
Sample Preparation
In the typical synthesis of MoP-NC, (NH4)6Mo7O24·4H2O (0.240 g), NH4H2PO4 (0.167 g), and CO(NH2)2 (2.000 g) were dissolved in 50 mL of deionized water and subjected to 15 min of sonication. Thereafter, the resulting solution was heated to 80 °C and magnetically stirred for 90 min, remained relatively closed throughout the reaction, and then was dried in a freeze dryer. The obtained white precursor powder was heated from room temperature to 900 °C at a rate of 5 °C/min under a N2 atmosphere for 120 min. In order to explore the influence of carbon source on material synthesis, MoP-C was prepared by using glucose instead of urea as carbon source. When no carbon source or phosphorus source was added, Bulk-MoP and Mo-NC were prepared, respectively.
Characterizations
The X-ray diffraction (XRD) information was collected on an X-ray diffraction (XRD, Bruker D8-Advance diffractometer with Cu Kαradiation (λ = 1.54056 Å)). The microstructure of the sample was obtained by a field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan). The TEM images were performed on a transmission electron microscopy (TEM, JEM-2100, JEOL, Japan). The chemical components were analyzed by an X-ray photoelectron spectroscopy (XPS) with Mg Kα as a monochromatic X-ray source.
Electrochemical Test
All electrochemical measurements were performed on an electrochemical workstation (CHI 660E Chenhua, Shanghai) equipped with a conventional three-electrode system of Pine Modulated Speed Rotator (PINE, USA). The Pt wire and the saturated calomel electrode (SCE) corresponded to the counter electrode and the reference electrode, respectively, and the glassy carbon electrode and the rotating disk device were connected as a working electrode. In addition, 1 M KOH electrolyte was provided for testing. The working electrode was prepared as follows: First, 5 mg of the catalyst was dissolved in a solution mixed with 350 μL of isopropanol, 650 μL of deionized water, and 50 μL of 5 wt% Nafion. Next, after the abovementioned mixed solution was ultrasonicated for 30 min, a uniformly dispersed ink was obtained. Finally, 10 μL of the ink was dropped on a glassy carbon electrode (diameter, 5 mm) for natural air-drying treatment, wherein the catalyst had an areal density of 0.485 mg cm−2. In order to better describe the performance parameters of the sample, we used a Pt/C catalyst (20 wt%) for comparison, and the preparation process was the same as that of the above working electrode. A sweep speed of 10 mV s−1 was used for linear sweep voltammetry (LSV) measurements. Tafel was obtained by fitting the appropriate region curve according to the Tafel equation, and the electrochemical stability was obtained by performing 1000 cycles with the sweep speed of 100 mV·S−1. The double-layer capacitance (Cdl) data is derived from cyclic voltammetry (CV), which is performed at the same voltage range (0.847–0.947 V vs RHE) at different scan rate ranges (20–200 mV). Electrochemical impedance spectroscopy (EIS) measurements were made at a constant potential amplitude of 10 mV over the default frequency range (1–105 Hz).
Implications of the Hypothesis
Figure 1a showed the XRD pattern of MoP-NC presents diffraction peaks at 27.95, 32.17, 43.15, 57.48, 57.95, 64.93, 67.03, 67.86, and 74.33, corresponding to the nine different crystal faces of MoP. SEM images of MoP-NC showed amorphous small particles microstructures (Fig. 1b). The particles gathered together to form small clusters, but there was still a certain gap between the clusters. This small and dense structure made MoP-NC both have considerable catalytic activity and good stability (Fig. 1c). It can be seen from TEM and high-resolution TEM (HRTEM) (Fig. 1d, e) that these nanoparticles showed distinct lattice fringes, such as lattice fringes from the (100) plane with a pitch of 0.28 nm. In addition, outside these well-defined lattice fringe regions were MoP-NC nanoparticle edges, which strongly supported the incorporation of MoP-NC nanoparticles into the carbon matrix. The corresponding EDS element mapping image (Fig. 1f–i) further verified the uniform distribution of the four elements in the product MoP-NC.
To further understand the elemental distribution of MoP-NC, XPS was characterized. In the Mo 3d spectral region, Mo contains two states of Mo3+ and Mo6+ (Fig. 2a). The presence of Mo 3d3/2 and Mo 3d5/2 in the Mo3+ state led to micro-vibration peaks at 231.5 and 228.2 ev, while the peaks of 235.5 and 232.4 ev were attributed to Mo 3d3/2 and Mo 3d5/2 in the Mo6+ state, because the surface of the MoP-NC material was inevitably oxidized in the air [24, 25]. In the P 2p region (Fig. 2b), micro-vibration peaks of 130.7 and 129.4 eV were assigned to P 2p1/2 and P 2p3/2, respectively, revealing the presence of P3− [26]. The peak at 133.9 eV can be attributed to PO43− [27]. In the C 1s XPS spectrum (Fig. 2c), the main peaks corresponding to the three chemical bonds were 228.7 eV (O-C=O), 284.8 eV (C-N/C=C), and 286.3 eV (C-C), respectively [28]. The appearance of C-N/C=N suggested that some of the carbon atoms in MoP-NC were replaced by nitrogen atoms to form N-doped carbon. In the spectrum of N 1s (Fig. 2d), three different nitrogen environments can be solved from this region, wherein the peaks of 398.4 and 402.1 eV with larger binding energies corresponded to the major amounts of pyridinium and the minor amount of quaternary nitrogen, respectively. In addition, a peak at 394.5 eV was designated as a combination of N and Mo 3p [29].
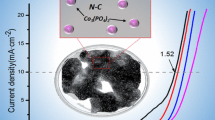
The electrocatalytic HER activity of MoP-NC in 1 M KOH (pH = 14) used typical three-electrode system with the sweep speed of 10 mV s−1. Since the properties of the prepared materials required comparative analysis, Mo-NC, Bulk-MoP, and MoP-C were also studied. Figure 3a depicted LSV curves. The addition of Pt/C and a blank glassy carbon electrode (Blank) made the entire curves look more hierarchical. The overpotential of MoP-NC at a current density of 10 mA cm−2 requires only 131 mV, which was significantly better than that of Mo-NC and Bulk-MoP. Furthermore, the LSV performance of MoP-C prepared from glucose instead of urea as a carbon source was also dwarfed by MoP-NC. It is worth noting that compared to HER in an acidic solution, the rate of H ion production during the decomposition of HER water in the alkaline was slower (about 2–3 orders of magnitude lower than acidic activity) and had a larger challenging [30,31,32]. Figure 3b showed the fitted Tafel graph equation: η = a + b log j, where b is the Tafel slope and j is the current density [33]. The Tafel slope of Pt/C is 58 mV dec−1, compared with Mo-NC (121 mV dec−1), Bulk-MoP (135 mV dec−1), and MoP-C (75 mV dec−1), MoP-NC had a lower parameter of only 66 mV dec−1, indicating that the HER catalytic kinetics for the MoP-NC electrode were faster. At the same time, we found that the MoP-NC in this work was quite competitive with the HER performance of the previously reported Mo-based composite/carbon electrocatalyst (Table 1). As can be seen from Table 1, most of the MoP-based materials were based on acidic conditions and were rarely tested under alkaline conditions [17,18,19, 22,23,24, 34,35,36,37]. In addition, some of them had been tested in both acidic and alkaline environments [38,39,40,41]. However, only amorphous carbon-coated MoP materials in these materials performed better in alkaline environments than in acidic environments. The reason why MoP-NC in our work can achieve good HER performance in alkaline environment was because urea was used as both a carbon source and a nitrogen source in the synthesis process, during which it also decomposed some gas. It slowed down the polymerization of N and C co-doped MoP, which played an excellent auxiliary synthesis role. The stability of the prepared materials was continuous CV with a scan rate of 100 mV s−1. After 1000 cycles, the LSV curve had a small current density loss compared to the initial value (Fig. 3c). Figure 3d showed the CV plots of MoP-NC, which was performed at the same voltage range (0.847–0.947 V vs RHE) at different scan rate ranges (20–200 mV). To further explore the double-layer capacitance (Cdl) of the material, the Cdl of a series of control groups was shown in Fig. 3e. The Cdl of Mo-NC, Bulk-MoP, and MoP-C were 0.8 mF cm−2, 60 μF cm−2 and 1.6 mF cm−2, respectively, while the Cdl of MoP-NC was 10.9 mF cm−2 which was much larger than the above materials. The suggestion indicated that MoP-NC had a higher active surface area. Furthermore, the conductivity of MoP-NC was evaluated by electrochemical impedance spectroscopy (EIS). Figure 3f showed the Nyquist diagram of the different catalysts. The charge transfer resistance of the MoP-NC catalyst was lower than that of other catalysts, which meant that the faster electron transfer ratio of the MoP-NC catalyst after N, C co-doping further enhanced the electrocatalytic performance of HER.
a LSV curves with scan rate of 10 mV s−1 at room temperature in 1 M KOH. b Tafel plots of the as-synthesized samples. c Stability of MoP-NC after 1000 cycles of voltammetry (CV) cycle. d CV plots of MoP-NC at a scan rate between 20 and 200 mV s−1. e Double-layer capacitor (Cdl) of Mo-NC, Bulk-MoP, MoP-NC, and MoP-C with a capacitor current of 0.1 V. f The EIS spectra of Mo-NC, Bulk-MoP, MoP-NC, and MoP-C
Conclusions
In summary, we synthesized the amorphous small particles of MoP-NC by a simple and efficient two-step method. Since the MoP nanoparticles were coated with carbon, they partially aggregated together. Fortunately, this structure does not drag the performance of the material itself but also contributes to its stability. This overall dispersion, locally aggregated small-particle material reached a current density of 10 mA cm−2 in 1 M KOH requiring only an overpotential of 131 mV, which is superior to the reported HER performance of a single molybdenum phosphide material in an alkaline environment. In addition, the material exhibited negligible performance degradation even after scanning 1000 CV cycles. Our results show that carbon-coated MoP can also overcome the alkaline environment to achieve excellent HER electrocatalytic activity and stability.
Availability of Data and Materials
All data are fully available without restriction.
Abbreviations
- CA:
-
Citric acid
- C dl :
-
The double-layer capacitance
- CV:
-
Cyclic voltammetry
- EIS:
-
Electrochemical impedance spectroscopy
- HER:
-
Hydrogen evolution reaction
- HRTEM:
-
High-resolution transition electronmicroscopy
- KOH:
-
Potassium hydroxide
- LSV:
-
Linear sweep voltammetry
- MoP:
-
Molybdenum phosphide
- MoP-NC:
-
N, C co-doped MoP
- SCE:
-
Saturated calomel electrode
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transition electronmicroscopy
- TMPs:
-
Transition metal phosphides
- TOP:
-
Trioctylphosphine
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray powder diffraction
References
Yuan W, Feng Y, Xie A et al (2016) Nitrogen-doped nanoporous carbon derived from waste pomelo peel as a metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 8(16):8704–8711
Jiao Y, Zheng Y, Jaroniec M et al (2015) Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem.Soc.Rew. 44(8):2060–2086
Chakrabartty S, Raj CR (2018) Mo2C@ NC nanowire bundle for efficient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energ. 43(42):19510–19520
Michalsky R, Zhang YJ, Peterson AA (2014) Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 4(5):1274–1278
Kumar R, Rai R, Gautam S et al (2017) Nano-structured hybrid molybdenum carbides/nitrides generated in situ for HER applications. J. Mater. Chem. A 5(17):7764–7768
Nørskov JK, Bligaard T, Logadottir A et al (2005) Trends in the exchange current for hydrogen evolution. J. Electrochem.l Soc 152(3):J23–J26
Pan Y, Liu Y, Liu C (2015) Phase-and morphology-controlled synthesis of cobalt sulfide nanocrystals and comparison of their catalytic activities for hydrogen evolution. Appl. Surf. Sci. 357:1133–1140
Merki D, Hu X (2011) Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energ. Environ. Sci. 4(10):3878–3888
Wang L, Liu X, Luo J et al (2017) Self-optimization of the active site of molybdenum disulfide by an irreversible phase transition during photocatalytic hydrogen evolution. Angew. Chem. 129:7718–7722
Ito Y, Cong W, Fujita T et al (2015) High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Edit. 54(7):2131–2136
Xiong J, Li J, Shi J et al (2018) In situ engineering of double-phase interface in Mo/Mo2C heteronanosheets for boosted hydrogen evolution reaction. ACS Energy Lett. 3(2):341–348
Pi M, Zhang D, Wang S et al (2018) Enhancing electrocatalytic hydrogen evolution of WP2 three-dimensional nanowire arrays via Mo doping. Materials Letters 213:315–318
Zhang Y, Li P, Yang X et al (2018) High-efficiency and stable alloyed nickel based electrodes for hydrogen evolution by seawater splitting. J. Alloy. Compd. 732:248–256
Yue Q, Wan Y, Sun Z et al (2015) MoP is a novel, noble-metal-free cocatalyst for enhanced photocatalytic hydrogen production from water under visible light[J]. J. Mater. Chem. A 3(33):16941–16947
Liang X, Zhang D, Wu Z et al (2016) The Fe-promoted MoP catalyst with high activity for water splitting. Appl. Catal. A-Gen. 524:134–138
Anjum MAR, Lee JS (2017) Sulfur and nitrogen dual-doped molybdenum phosphide nanocrystallites as an active and stable hydrogen evolution reaction electrocatalyst in acidic and alkaline media. ACS Catal. 7(4):3030–3038
Yang J, Zhang F, Wang X et al (2016) Porous molybdenum phosphide nano-octahedrons derived from confined phosphorization in UIO-66 for efficient hydrogen evolution. Angew. Chem. Int. Edit. 55(41):12854–12858
Xing Z, Liu Q, Asiri AM et al (2014) Closely interconnected network of molybdenum phosphide nanoparticles: a highly efficient electrocatalyst for generating hydrogen from water. Adv. mater. 26(32):5702–5707
McEnaney JM, Crompton JC, Callejas JF et al (2014) Amorphous molybdenum phosphide nanoparticles for electrocatalytic hydrogen evolution. Chem.Mater. 26(16):4826–4831
Wang Y, Nie Y, Ding W et al (2015) Unification of catalytic oxygen reduction and hydrogen evolution reactions: highly dispersive Co nanoparticles encapsulated inside Co and nitrogen co-doped carbon. Chem. Commun. 51(43):8942–8945
Cui W, Liu Q, Xing Z et al (2015) MoP nanosheets supported on biomass-derived carbon flake: One-step facile preparation and application as a novel high-active electrocatalyst toward hydrogen evolution reaction. Appl. Catal. B-Environ. 164:144–150
Wu Z, Wang J, Liu R et al (2017) Facile preparation of carbon sphere supported molybdenum compounds (P, C and S) as hydrogen evolution electrocatalysts in acid and alkaline electrolytes. Nano Energy 32:511–519
Gao S, Liu Y, Li GD et al (2016) General urea-assisted synthesis of carbon-coated metal phosphide nanoparticles for efficient hydrogen evolution electrocatalysis. Electrochim. Acta 199:99–107
Xiao P, Sk MA, Thia L et al (2014) Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energ. Environ. Sci. 7(8):2624–2629
Wang T, Du K, Liu W et al (2015) Enhanced electrocatalytic activity of MoP microparticles for hydrogen evolution by grinding and electrochemical activation. J. Mater. Chem. A 3(8):4368–4373
Zhang X, Liu Y, Xiong Q et al (2017) Vapour-phase hydrothermal synthesis of Ni2P nanocrystallines on carbon fiber cloth for high-efficiency H2 production and simultaneous urea decomposition. Electrochim. Acta 254:44–49
Ojha K, Sharma M, Kolev H et al (2017) Reduced graphene oxide and MoP composite as highly efficient and durable electrocatalyst for hydrogen evolution in both acidic and alkaline media. Catal. Sci. Technol. 7(3):668–676
Pu Z, Amiinu IS, Liu X et al (2016) Ultrastable nitrogen-doped carbon encapsulating molybdenum phosphide nanoparticles as highly efficient electrocatalyst for hydrogen generation. Nanoscale 8(39):17256–17261
Yan H, Xie Y, Jiao Y et al (2018) Holey reduced graphene oxide coupled with an Mo2N–Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 30(2):1704156
Wang L, Liu X, Zhang Q et al (2019) Quasi-one-dimensional Mo chains for efficient hydrogen evolution reaction. Nano Energy 61:194–200
Wang S, Zhang L, Xia Z et al (2012) BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Edit. 51(17):4209–4212
Zheng Y, Jiao Y, Vasileff A et al (2018) The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Edit. 57(26):7568–7579
Yan D, Li F, Xu Y et al (2018) Three-dimensional reduced graphene oxide–Mn3O4 nanosheet hybrid decorated with palladium nanoparticles for highly efficient hydrogen evolution. Int. J. Hydrogen Energy 43(6):3369–3377
Jia J, Zhou W, Li G et al (2017) Regulated synthesis of Mo sheets and their derivative MoX sheets (X: P, S, or C) as efficient electrocatalysts for hydrogen evolution reactions. ACS appl. Mater. inter. 9(9):8041–8046
Chi JQ, Gao WK, Lin JH et al (2018) Nitrogen, phosphorus dual-doped molybdenum-carbide/molybdenum-phosphide-@-carbon nanospheres for efficient hydrogen evolution over the whole pH range. J. colloid interf. Sci. 513:151–160
Guo Z, Liu P, Liu J et al (2018) Neural network inspired design of highly active and durable N-doped carbon interconnected molybdenum phosphide for hydrogen evolution reaction. ACS Appl. Energy Mater. 1(10):5437–5445
Zhang L, Yang Y, Ziaee MA et al (2018) Nanohybrid of carbon quantum dots/molybdenum phosphide nanoparticle for efficient electrochemical hydrogen evolution in alkaline medium. ACS appl. Mater. Inter. 10(11):9460–9467
Huang Y, Song X, Deng J et al (2019) Ultra-dispersed molybdenum phosphide and phosphosulfide nanoparticles on hierarchical carbonaceous scaffolds for hydrogen evolution electrocatalysis. Appl. Catal. B-Environ. 245:656–661
Wu Z, Wang J, Zhu J et al (2017) Highly efficient and stable MoP-RGO nanoparticles as electrocatalysts for hydrogen evolution. Electrochim. Acta 232:254–261
Zhang LN, Li SH, Tan HQ et al (2017) MoP/Mo2C@ C: a new combination of electrocatalysts for highly efficient hydrogen evolution over the entire pH range. ACS Appl. Mater. Inter 9(19):16270–16279
Liu X, Zhang L, Li M et al (2018) Tandem MoP nanocrystals with rich grain boundaries for efficient electrocatalytic hydrogen evolution. Chem. Commun 54(20):2502–2505
Acknowledgements
Not applicable
Funding
This work was financed by National Natural Science Foundation of China (51502005) and the Quality Engineering Project of Colleges and Universities of Anhui Province (2017sxzx15, 2018zygc012).
Author information
Authors and Affiliations
Contributions
LC and QH prepared the compounds and tested the electrochemical performance. Surface topography and x-ray diffraction was investigated by JL and RT. WZ did some ancillary work. YL participated in the design and coordination of this study. The calculations were carried out by LC who also wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, Y., Cai, L., Huang, Q. et al. Highly Efficient Synthesis of Carbon-Based Molybdenum Phosphide Nanoparticles for Electrocatalytic Hydrogen Evolution. Nanoscale Res Lett 15, 6 (2020). https://doi.org/10.1186/s11671-020-3246-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-020-3246-x