Abstract
Background
Elimination of hazardous phenolic compounds using laccases has gained attention during recent decades. The present study was designed to evaluate the ability of the purified laccase from Paraconiothyrium variabile (Pv L) for elimination of phenol and the endocrine disrupting chemical bisphenol A. Effect of laccase activity, pH, and temperature on the enzymatic removal of the mentioned pollutants were also investigated.
Results
After 30 min treatment of the applied phenolic pollutants in the presence of Pv L (5 U/mL), 80% of phenol and 59.7% of bisphenol A was removed. Increasing of laccase activity enhanced the removal percentage of both pollutants. The acidic pH of 5 was found to be the best pH for elimination of both phenol and bisphenol A. Increasing of reaction temperature up to 50°C enhanced the removal percentage of phenol and bisphenol A to 96.3% and 88.3%, respectively.
Conclusions
To sum up, the present work introduced the purified laccase of P. variabile as an efficient biocatalyst for removal of one of the most hazardous endocrine disruptor bisphenol A.
Similar content being viewed by others
Introduction
Phenol is one of the most environmentally hazardous pollutants which increasingly been applied by industries like petrochemical, coking, plastics, paper and oil refineries, as well as phenolic resin industries[1, 2]. Poor solubility and biodegradability, long term ecological damage and high toxicity of phenol and its derivatives led to calls by Environmental Protection Agency (EPA) for lowering phenol concentration in the wastewater to less than 1 mg/mL before discharging them into the water reservoirs[3]. The estrogenic action and impacts of the bisphenol A (4,4′-isopropylidenediphenol, BPA), an industrial chemical which is widely used for the synthesis of polycarbonate plastics and epoxy resins, on female reproductive tract introduced BPA as an endocrine disrupting chemical (EDC)[4, 5]. The role of BPA exposure in development of prostate and breast cancers, reduction of human sperm counts, alteration of immune functions, prevalence of obesity and decrease fertility in fish and mammals has been demonstrated by some studies[5, 6]. So, various physicochemical processes like activated carbon adsorption, solvent extraction, chemical oxidation and electrochemical methods have been developed to remove phenolic compounds from wastewaters[5, 7–13]. However, wide applications of some of these remediation strategies have been limited due to problems such as high cost, low efficiency, and generation of toxic by-products[14, 15]. On the other hand, advantages of biological techniques including biodegradation of xenobiotics using living microorganisms like algae[16], bacteria and fungi and/or their purified oxidizing enzymes both in free[17, 18] and immobilized form[19–22] as well as biosorption of organic pollutants[1, 23] introduced this field as a novel area for removal of hazardous compounds.
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) are copper containing oxidases catalyzing the oxidation of a wide range of aromatic substrates including phenol derivatives, benzenethiols, polyphenols[24] and polycyclic aromatic hydrocarbons (PAHs)[25]. In recent decades, laccase-producing microorganisms, especially white-rot fungi[25, 26] have been employed for biological treatment of different pollutants[27]. Biodegradation of brominated phenols using cultures and laccase of Trametes versicolor was investigated by Uhnakova et al.[26]. Zhang et al.[24] studied on degradation of 2,4-dichlorophenol (2,4-DCP), 4-chlorophenol (ρ-CP), and 2-chlorophenol catalyzed by laccase from Coriolus versicolor.
The aim of the present study was to investigate on the ability of the purified laccase from the ascomycete Paraconiothyrium variabile, a newly isolated laccase-producing ascomycete from soil, for elimination of phenol and bisphenol A. Effect of parameters such as laccase activity, pH, and temperature on removal of pollutants was also studied.
Materials and methods
Laccase and chemicals
The produced laccase of P. variabile was purified from fungal culture broth based on the method described by Forootanfar et al.[28] and applied in pollutant removal experiments. Phenol, bisphenol A (BPA), and 4-aminoantipyrine (4-AAP) were purchased from Merck (Darmstadt, Germany). 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) was obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.
Determination of laccase activity
Oxidation of ABTS as a laccase substrate was used to determine the laccase activity[29, 30]. In brief, 0.5 mL of enzyme sample was added to 0.5 mL of ABTS solution (5 mM in 0.1 M citrate buffer, pH 4.5) and incubated at 37°C and 120 rpm for 10 min. Change in absorbance at 420 nm was monitored by a UV/vis spectrophotometer (UVD 2950, Labomed, Culver City, USA) and the laccase activity was calculated using the molar extinction coefficient of ABTS (ϵ420 = 36,000 M-1 cm-1). One unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol of substrate per minute[31].
Removal studies
In order to study on the ability of the purified laccase for elimination of phenolic pollutants, the reaction mixture (final volume of 3 mL) was prepared as follow: 1 mL of phenol or BPA solution (final concentration of 4 mM) was added to 1 mL citrate buffer (20 mM pH 5) followed by introducing of the purified laccase (final concentration of 5 U/mL) to the reaction mixture and incubation at 35°C and 50 rpm for 40 min. Samples were taken every 10 min and analyzed for remaining concentration of phenolic pollutants. The negative control was designed by inserting of heat-inactivated laccase to the reaction mixture. Each experiment was performed in triplicate and mean of the obtained results were reported.
Determination of phenol and bisphenol A concentration
Concentration of phenolic pollutants was measured using a colorimetric assay in presence of 4-AAP as a primary amine[6]. Briefly, the reaction mixture was prepared by addition of 700 μL of phosphate buffer (0.1 M pH 8), 300 μL of laccase-treated phenol or BPA sample, 10 μL of 4-AAP (0.1 M) and 10 μL potassium ferricyanide solution (0.2 M) followed by incubation at 25°C and 100 rpm for 15 min. The absorbance of the reaction mixture was then measured at 506 nm and the phenol or BPA concentration was determined from the obtained standard curve.
Effect of laccase activity on the removal of phenolic pollutants
The effect of laccase activity on the removal of phenol or BPA was studied by adding of enzyme solution (1, 5, 10 and 20 U/mL in citrate buffer 20 mM pH 5) to the phenolic pollutant solutions (final concentration of 4 mM) and incubation at 35°C and 50 rpm for 30 min. The reaction mixture was then analyzed for remained phenolic pollutant concentration as described above.
The effect of pH on laccase-mediated removal
After adjusting the initial pH of the phenolic pollutant solution (final concentration of 4 mM) using 20 mM citrate-phosphate buffer between 3–7, the purified laccase (5 U/mL) was added to the reaction mixture and incubated at 35°C and 50 rpm for 30 min. The pollutant concentration was then monitored as previously described.
The influence of temperature on enzymatic removal
The effect of temperature on enzymatic elimination of pollutants was studied by incubating 4 mM of the phenolic pollutant solution (in citrate buffer 20 mM pH 5) in the presence of laccase (5 U/mL) at temperature range of 30–70°C.
Results and discussion
Pollutant elimination by the purified laccase
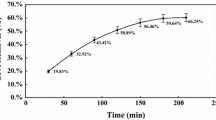
As shown in the time course of phenolic pollutant elimination curves (Figure 1) the purified Pv L removed 29.6% and 44.3% of BPA and phenol, respectively after 10 min treatment. After 30 min, the removal percentages increased by 59.7% and 80% in the case of BPA and phenol, respectively (Figure 1). No elimination was detected in the case of negative controls. The enzymatic removal of phenol and related hazardous compounds especially the endocrine disruptor compound (bisphenol A) discharged from industrial effluents into the environment has received more attention during recent decades[32, 33]. Potential of Pv L for removing of a wide range of chemicals including chlorophenols, synthetic dyes and benzodiazepines have been shown in recent studies[14, 15, 22, 27]. The present study showed that both applied phenolic pollutants (initial concentration of 4 mM) was eliminated using the purified laccase of P. variabile after 30 min incubation. Kurniawati et al.[33] reported about removal of phenol (initial concentration of 500 μM) after 6 h incubation in presence of the purified laccase from Trametes versicolor. The purified laccase of Fusarium incarnatum UC-14 hosted in the reversed micelle was able to remove 91.43% of bisphenol A (200 ppm) after 2 h incubation[5].
The effect of laccase activity on removal of phenolic pollutants
The pattern of removal of pollutants by increasing of laccase activity from 1 U/mL to 20 U/mL is illustrated in Figure 2. In the present study, it was showed that elimination of both phenol and bisphenol A increased when laccase activity was enhanced. Same results was observed in study of Okazaki et al.[34], which indicated that increasing of laccase concentration (originated from Coriolus versicolor) from 0 to 50 μg/mL enhanced BPA removal to 100%. Application of the purified laccase from Trichoderma atroviride (0.3 U/mL) for elimination of phenolic pollutants including 2,4-dichlorophenoxyacetic acid, 4-chlorophenol, o-cresol or and catechol led to 21%, 28%, 100% and 100% removal of pollutants, respectively after 24 h incubation[35].
The influence of pH on pollutant removal
As illustrated in Figure 3, both of studied pollutants represented maximum removal percentages (77.7% and 59.3% in the case of phenol and BPA, respectively) at pH 5, which was previously introduced as the optimum pH for the activity of the purified laccase from P. variabile[28]. Majority of the fungal laccases optimally act in acidic pH[36]. In the study of Chhaya and Gupte[5], who evaluated the activity of laccase from Fusarium incarnatum UC-14 toward BPA, the pH value of 6 was introduced as optimal pH for removal of bisphenol A. study of Liu et al.[37] showed that acidic environment (pH 6) was the best condition for phenol removal using the recombinant laccase of T. versicolor.
The effect of temperature on laccase-mediated pollutant removal
The optimum temperature for the elimination of phenol (96.3% removal) and BPA (88.3% removal) was found to be 50°C (Figure 4). This temperature is the optimum temperature of Pv L activity determined by Forootanfar et al.[28]. At elevated temperature of 60°C the removal percentage of phenol and BPA in presence of Pv L dropped to 47% and 48.3%, respectively. These results were in agreement with the findings of Kurniawati and Nicell[33], who determined maximum laccase-assisted phenol elimination between 40°C and 50°C and a sharp decrease in pollutant removal was observed above 60°C. In the study of Kim et al.[4] maximum of bisphenol A degradation (67%) using the laccase of Trametes versicolor (0.15 U/mL) was achieved at temperature of 45°C. Most of the fungal laccases maximally act in the temperature range of 50–70°C, although the maximum activity of laccase from G. lucidum was at 25°C[36].
Conclusion
The purified laccase of P. variabile applied for removal of phenol and bisphenol A. Pv L was efficiently eliminated both applied pollutants after 30 min treatment. Maximum of removal percent in the case both phenolic pollutants was obtained at optimum pH and temperature of the Pv L. To sum up, the results of the present investigation candidate the purified Pv L for removal of phenolic pollutants. However, more study should be conducted to find out about probable produced metabolites.
References
Bayramoglu G, Gursel I, Tunali Y, Arica MY: Biosorption of phenol and 2-chlorophenol by Funalia trogii pellets. Bioresour Technol 2009, 100: 2685–2691.
Ryan D, Leukes W, Burton S: Improving the bioremediation of phenolic wastewaters by Trametes versicolor . Bioresour Technol 2007, 98: 579–587.
Busca G, Berardinelli S, Resini C, Arrighi L: Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 2008, 160: 265–288.
Kim Y-J, Nicell JA: Impact of reaction conditions on the laccase-catalyzed conversion of bisphenol A. Bioresour Technol 2006, 97: 1431–1442.
Chhaya U, Gupte A: Possible role of laccase from Fusarium incarnatum UC-14 in bioremediation of Bisphenol A using reverse micelles system. J Hazard Mater 2013, 254–255: 149–156.
Modaressi K, Taylor KE, Bewtra JK, Biswas N: Laccase-catalyzed removal of bisphenol-A from water: protective effect of PEG on enzyme activity. Water Res 2005, 39: 4309–4316.
Inoue M, Masuda Y, Okada F, Sakurai A, Takahashi I, Sakakibara M: Degradation of bisphenol A using sonochemical reactions. Water Res 2008, 42: 1379–1386.
Mahvi AH, Maleki A, Alimohamadi M, Ghasri A: Photo-oxidation of phenol in aqueous solution: toxicity of intermediates. Korean J Chem Eng 2007, 24: 79–82.
Maleki A, Mahvi AH, Mesdaghinia A, Naddafi K: Degradation and toxicity reduction of phenol by ultrasound waves. Bull Chem Soc Ethiopia 2007, 21: 33–38.
Mahvi AH, Maleki A: Photosonochemical degradation of phenol in water. Desalin Water Treat 2010, 20: 197–202.
Bazrafshan E, Biglari H, Mahvi AH: Phenol removal by electrocoagulation process from aqueous solutions. Fresenius Environ Bull 2012, 21: 364–371.
Bazrafshan E, Mostafapour FK, Mahvi AH: Phenol removal from aqueous solutions using pistachio-nut shell ash as a low cost adsorbent. Fresenius Environ Bull 2012, 21: 2962–2968.
Maleki A, Mahvi AH, Alimohamadi M, Ghasri A: Advanced oxidation of phenol by ultraviolet irradiation in aqueous system. Pakistan J Biol Sci 2006, 9: 2338–2341.
Forootanfar H, Moezzi A, Aghaie-Khozani M, Mahmoudjanlou Y, Ameri A, Niknejad F, Faramarzi MA: Synthetic dye decolorization by three sources of fungal laccase. Iranian J Environ Health Sci Eng 2012, 9: 27.
Forootanfar H, Movahednia MM, Yaghmaei S, Tabatabaei-Sameni M, Rastegar H, Sadighi A, Faramarzi MA: Removal of chlorophenolic derivatives by soil isolated ascomycete of Paraconiothyrium variabile and studying the role of its extracellular laccase. J Hazard Mater 2012, 209–210: 199–203.
Forootanfar H, Shakibaie M, Bagherzadeh Z, Aghaie-Khozani M, Nafissi-Varcheh N, Monsef-Esfahani HR, Faramarzi MA: The removal of ρ-chlorophenol in aqueous cultures with free and alginate-immobilized cells of the microalga Tetraselmis suecica . J Appl Phycol 2013, 25: 51–57.
Gholami-Borujeni F, Mahvi AH, Nasseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M: Enzymatic treatment and detoxification of acid orange 7 from textile wastewater. Appl Biochem Biotechnol 2011, 165: 1274–1284.
Gholami-Borujeni F, Faramarzi MA, Nejatzadeh-Barandozi F, Mahvi AH: Oxidative degradation and detoxification of textile azo dye by horseradish peroxidase enzyme. Fresen Environ Bull 2013, 22: 739–744.
Gholami-Borujeni F, Mahvi AH, Naseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M: Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res J Chem Environ 2011, 15: 217–222.
Dehghanifard E, Jafari AJ, Kalantary RR, Mahvi AH, Faramarzi MA, Esrafili A: Biodegradation of 2,4-dinitrophenol with laccase immobilized on nano-porous silica beads. J Environ Health Sci Eng 2013, 10: 25.
Sadighi A, Faramarzi MA: Congo red decolorization by immobilized laccase through chitosan nanoparticles on the glass beads. J Taiwan Inst Chem Eng 2013, 44: 156–162.
Mogharabi M, Nassiri-Koopaei N, Bozorgi-Koushalshahi M, Nafissi-Varcheh N, Bagherzadeh G, Mohammad Ali Faramarzi MA, Faramarzi MA: Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinorg Chem Appl 2012, 2012: 6. Article ID 823830
Lu Y, Yan L, Wang Y, Zhou S, Fu J, Zhang J: Biodegradation of phenolic compounds from coking wastewater by immobilized white rot fungus Phanerochaete chrysosporium . J Hazard Mater 2009, 165: 1091–1097.
Zhang J, Liu X, Xu Z, Chen H, Yang Y: Degradation of chlorophenols catalyzed by laccase. Int Biodeter Biodegr 2008, 61: 351–356.
Ting WTE, Yuan SY, Wu SD, Chang BV: Biodegradation of phenanthrene and pyrene by Ganoderma lucidum . Int Biodeter Biodegr 2011, 65: 238–242.
Uhnakova B, Petrickova A, Biedermann D, Homolka L, Vejvoda B, Bendnar P, Papouskova B, Sulk M, Martinkova L: Biodegradation of brominated aromatics by cultures and laccase of Trametes versicolor . Chemosphere 2009, 76: 826–832.
Ostadhadi-Dehkordi S, Tabatabaei-Sameni M, Forootanfar H, Kolahdouz S, Ghazi-Khansari M, Faramarzi MA: Degradation of some benzodiazepines by a laccase-mediated system in aqueous solution. Bioresour Technol 2012, 125: 344–347.
Forootanfar H, Faramarzi MA, Shahverdi AR, Tabatabaei Yazdi M: Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile . Bioresour Technol 2011, 102: 1808–1814.
Faramarzi MA, Forootanfar H: Biosynthesis and characterization of gold nanoparticles produced by laccase from Paraconiothyrium variabile . Colloids Surf B 2011, 87: 23–27.
Aghaie-Khouzania M, Forootanfar H, Moshfegh M, Khoshayand MR, Faramarzi MA: Decolorization of some synthetic dyes using optimized culture broth of laccase producing ascomycete Paraconiothyrium variabile. Biochem Eng J 2012, 60: 9–15.
Alberts JF, Gelderblom WCA, Botha A, Vanzyl WH: Degradation of aflatoxin B1 by fungal laccase enzymes. Int J Food Microbiol 2009, 135: 47–52.
Telke AA, Kalyani DC, Jadhav UU, Parshetti GK, Govindwar SP: Purification and characterization of an extracellular laccase from a Pseudomonas sp. LBC1 and its application for the removal of bisphenol A. J Mol Catal B 2009, 61: 252–260.
Kurniawati S, Nicell JA: Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour Technol 2008, 99: 7825–7834.
Okazaki S-Y, Michizoe J, Goto M, Shintaro Furusaki S, Wariishi H, Tanak H: Oxidation of bisphenol A catalyzed by laccase hosted in reversed micelles in organic media. Enzyme Microb Technol 2002, 31: 227–232.
Chakroun H, Mechichi T, Martinez MJ, Dhouib A, Sayadi S: Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride : application on bioremediation of Phenolic compounds. Process Biochem 2010, 45: 507–513.
Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI: "Blue" laccases. Biochem Mosc 2007, 72: 1136–1150.
Liu Z-F, Zeng G-M, Zhong H, Yuan X-Y, Fu H-Y, Zhou M-F, Ma X-L, Li H, Li J-B: Effect of dirhamnolipid on the removal of phenol catalyzed by laccase in aqueous solution. World J Microbiol Biotechnol 2012, 28: 175–181.
Acknowledgement
This work was financially supported by a grant from Center for Water Quality Research, Institute for Environmental Research, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZA carried out the elimination studies of phenol and bisphenol A. Production and purification of laccase from P. variabile culture broth was performed by SR and HF, respectively. AHM contributed in writing of the manuscript, elimination studies and analyzing of data. MAF involved in purchasing of required materials and instruments, designing of removal experiments, analyzing of data and reviewing of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Asadgol, Z., Forootanfar, H., Rezaei, S. et al. Removal of phenol and bisphenol-A catalyzed by laccase in aqueous solution. J Environ Health Sci Engineer 12, 93 (2014). https://doi.org/10.1186/2052-336X-12-93
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2052-336X-12-93