Abstract
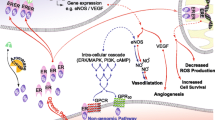
Atherosclerosis is the main cause of death in men and women. This so-called “hardening of the arteries” results from advanced atherogenesis, the accumulation and death of subendothelial fat-laden macrophages (vascular plaque). The macrophages are attracted as the result of signals from injured vessels recruiting and activating cells to quell the injury by inflammation. Among the recruited cells are circulating monocytes that may be captured by the formation of neural cell adhesion molecule (nCAM) tethers between the monocytes and vascular endothelium; the tethers are dependent on electrostatic binding between distal segments of apposed nCAM molecules. The capture of monocytes is followed by their entry into the subendothelial area as macrophages, many of which will remain and become the fat-laden foam cells in vascular plaque. Neural cell adhesion molecules are subject to sialylation that blocks their electrostatic binding. We showed that estradiol-induced nCAM sialylases are present in vascular endothelial cells and tested whether sex steroid pretreatment of human vascular endothelium could inhibit the capture of monocytes. Using in vitro techniques, pretreatment of human arterial endothelial cells with estradiol, testosterone, dehy-droepiandrosterone and dihydrotestosterone all induced sialylation of endothelial cells and, in a dose-response manner, reduced the capture of monocytes. Steroid hormones are protective against atherogenesis and its sequellae. Sex steroid depletion is associated with atherosclerosis. Based on this knowledge plus our results using sex steroid pretreatment of endothelial cells, we propose that the blockade of the initial step in atherogenesis by sex steroid-induced nCAM sialylation may be crucial to hormonal prevention of atherosclerosis.
Similar content being viewed by others
References
Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587.
Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351(9113):1425–427.
Colditz GA, Willet WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–1110.
Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122(11):1016–1022.
Kalantaridou SN, Naka KK, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89(8):3907–3913.
Perez-Lopez FR, Chedraui P, Gilbert JJ, Perez-Roncero G. Cardiovascular risk in menopausal women and prevalent related co-morbid conditions: facing the post-Women’s health initiative era. Fertil Steril. 2009;92(4):1171–1186.
Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98(1):83–90.
Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981;139(1):47–51.
Shufelt CL, Merz CN, Prentice RL, et al. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women’s Health Initiative Observational Study. Menopause. 2014;21(3):260–266.
Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313(17):1044–1049.
Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933–941.
Manson JE. Postmenopausal hormone therapy and atherosclerotic disease. Am Heart J. 1994;128(6 pt 2):1337–1343. Review.
Ge QS, Tian QJ, Tseng H, Naftolin F. Development of low-dose reproductive hormone therapies in China. Gynecol Endocrinol. 2006;22(11):110.
Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342–353.
Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women in The Kronos Early Estrogen Prevention Study (KEEPS): a randomized controlled trial. Ann Intern Med. 2014;161(4):249–260.
Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early Vs late intervention trial with estradiol. Circulation. 2014;130(11):A13283.
Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary artery calcification. N Engl J Med. 2007;256(11):2591–2602.
Tan O, Harman SM, Naftolin F. What can we learn from design faults in the women’s health initiative randomized clinical trial? Bull NYU Hosp Jt Dis. 2009;67(2):226–229.
Choi S, Steinberg E, Lee H, Naftalin F. The timing hypothesis remains a valid explanation of different cardioprotective effects of menopausal hormone treatment. Menopause. 2011;18(2):230–236.
Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;11(7):CD004143.
Garcia M, Miller VM, Gulati M, et al. Focused cardiovascular care for women: the need and role in clinical practice. Mayo Clin Proc. 2016;91(2):226–240.
Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–218.
Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373.
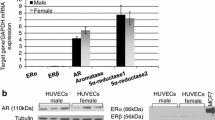
Diano S, Horvath TL, Mor G, et al. Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause. 1999;6(1):21–28.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874.
Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015; 116(2): 307-311.
Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372.
Berliner JA, Territo MC, Sevanian A, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85(4):1260–1266.
Berliner JA, Navab M, Fogelman AM, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91(9):2488–2496.
Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102(1):145–152.
Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191(1):189–194.
Galkina E, Ley E. Vascular adhesion molecules in atherosclerosis. Atherioscler Thromb Vas Biol. 2007;27(11):2292–2301.
Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain Res Rev. 2007;56(1):101–118.
Mor G, Nilsen J, Horvath T, et al. Estrogen and microglia: a regulatory system that affects the brain. J Neurobiol. 1999;40(4):484–496.
Park H, Pagan L, Tan O, et al. Estradiol regulates expression of polysialated neural cell adhesion molecule by human vascular endothelial cells. Reprod Sci. 2010;17(12):1090–1098.
Kiss JZ, Rougon G. Cell biology of polysialic acid. Curr Opin Neurobiol. 1997;7(5):640–646.
Rougon G. Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol. 1993;61(2):197–207.
Curatola AM, Huang K, Naftolin F. Dehydroepiandrosterone (DHEA) inhibition of monocyte binding by vascular endothelium is associated with sialylation of neural cell adhesion molecule. Reprod Sci. 2012;19(1):86–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naftolin, F., Mehr, H. & Fadiel, A. Sex Steroids Block the Initiation of Atherosclerosis. Reprod. Sci. 23, 1620–1625 (2016). https://doi.org/10.1177/1933719116674078
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116674078