Abstract
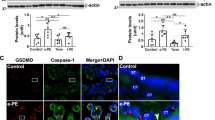
The soluble fms-like tyrosine kinase 1 (sFlt-1), known to be increased in the serum of preeclamptic patients, is a relevant factor in causing maternal symptoms like hypertension and proteinuria. In this study, we aimed to reveal whether hypoxia is a cause of increased sFlt-1 levels and inflammation markers in vivo and whether these symptoms can be attenuated by interleukin 6 (IL-6) depletion. For this purpose, pregnant wild-type (wt) mice or IL-6−/− mice on embryonic day 16 were placed under either normoxic (20.9% oxygen) or hypoxic (6% oxygen) conditions for 6 hours. This led to a rise of sFlt-1 levels in maternal serum, independent of the IL-6 status of the dam. Increased maternal sFlt-1 serum levels were, however, not due to an increase in sFlt-1 messenger RNA levels in the placenta. Moreover, there was no increase in inflammatory markers in neither wt mice nor IL-6−/− mice. This suggests that hypoxia alone does not contribute to the induction of an inflammatory placenta. Also, the hypoxia-induced rise in sFlt-1 levels seems not to be mediated by IL-6 in vivo.
Similar content being viewed by others
References
Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62(3):739–747.
Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998;91(9):3300–3307.
James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12(2):137–144.
Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285.
Ilekis JV, Reddy UM, Roberts JM. Preeclampsia—a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007; 14(6):508–523.
ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–167.
Guzin K, Tomruk S, Tuncay YA, et al. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch Gynecol Obstet. 2005;272(4):283–288.
Naicker T, Khedun SM, Moodley J, Pijnenborg R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Gynecol Scand. 2003;82(8):722–729.
Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7): 672–683.
Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29(2): 151–162.
Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol. 2008;199(5):566. e561–e511.
Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999; 163(6):3491–3495.
Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12(6):428–432.
Lockwood CJ, Yen CF, Basar M, et al. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172(6):1571–1579.
Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82(5): 1582–1588.
Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164(3):1049–1061.
Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine. 2009;47(1):56–60.
Peltier MR, Gurzenda EM, Murthy A, et al. Can oxygen tension contribute to an abnormal placental cytokine milieu? Am J Reprod Immunol. 2011;66(4):279–285.
Rajakumar A, Powers RW, Hubel CA, et al. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta. 2009; 30(1):25–34.
George EM, Cockrell K, Adair TH, Granger JP. Regulation of sFlt-1 and VEGF secretion by adenosine under hypoxic conditions in rat placental villous explants. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1629-R1633.
Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1085-1093.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12): 5510–5514.
Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57(3):505–514.
Zhou CC, Irani RA, Dai Y, et al. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186(10):6024–6034.
Trollmann R, Strasser K, Keller S, et al. Placental HIFs as markers of cerebral hypoxic distress in fetal mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1973-R1981.
Rother E, Kuschewski R, Alcazar MA, et al. Hypothalamic JNK1 and IKKbeta activation and impaired early postnatal glucose metabolism after maternal perinatal high-fat feeding. Endocrinology. 2012;153(2):770–781.
Hegen M, Gaestel M, Nickerson-Nutter CL, Lin LL, Telliez JB. MAPKAP kinase 2-deficient mice are resistant to collagen-induced arthritis. J Immunol. 2006;177(3):1913–1917.
Yang J, Rosen SD, Bendele P, Hemmerich S. Induction of PNAd and N-acetylglucosamine 6-O-sulfotransferases 1 and 2 in mouse collagen-induced arthritis. BMC Immunol. 2006;7:12.
Huckle WR, Roche RI. Post-transcriptional control of expression of sFlt-1, an endogenous inhibitor of vascular endothelial growth factor. J Cell Biochem. 2004;93(1): 120–132.
Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005; 365(9461):785–799.
Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24:147–158.
Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat Diagn. 2009;29(5):464–470.
Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891.
He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol. 1999;13(4):537–545.
Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta. 2010;31(6): 512–518.
Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90(22): 10705–10709.
Herse F, Fain JN, Janke J, et al. Adipose tissue-derived soluble fms-like tyrosine kinase 1 is an obesity-relevant endogenous paracrine adipokine. Hypertension. 2011;58(1):37–42.
Lee MC, Wei SC, Tsai-Wu JJ, Wu CH, Tsao PN. Novel PKC signaling is required for LPS-induced soluble Flt-1 expression in macrophages. J Leukoc Biol. 2008;84(3):835–841.
Xiong Y, Liebermann DA, Tront JS, et al. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol. 2009;220(3):632–639.
van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997; 176(2):439–444.
Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Appel, S., Turnwald, EM., Ankerne, J. et al. Hypoxia-Mediated Soluble Fms-Like Tyrosine Kinase 1 Increase Is Not Attenuated in Interleukin 6-Deficient Mice. Reprod. Sci. 22, 735–742 (2015). https://doi.org/10.1177/1933719114557898
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114557898