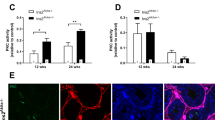
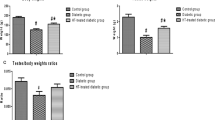
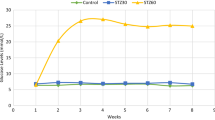
Abstract
Diabetes is increasingly becoming a major cause of large-scale morbidity and mortality. Diabetes-induced oxidative stress alters numerous intracellular signaling pathways. Although testicular dysfunction is a major concern in diabetic men, the mechanistic alterations in the testes that lead to hypogonadism are not yet clear. Oxidative mitochondrial DNA damage, as indicated by 7,8-dihydro-8-oxo-2′-deoxyguanosine, and phosphorylation of p53 at ser315 residue (p-p53ser315) increased in a stage- and cell-specific manner in the testes of rats that were diabetic for 1 month (DM1). Prolongation of diabetes for 3 months (DM3) led to an increase in nuclear oxidative DNA damage in conjunction with a decrease in the expression of p-p53ser315. The nuclei of pachytene and preleptotene spermatocytes, steps 1, 11, and 12 spermatids, secondary spermatocytes and the Sertoli cells, and the meiotic figures showed an increase in the expression of p-p53ser315. An increase in the expression of a downstream target of p53 and protein 21cyclin-dependent kinase interacting protein 1/wild-type p53-activated factor 1 (p21CIP1/Waf1) in both diabetic groups did not show any time-dependent effects but occurred concurrent with an upregulation of p-p53ser315 in DM1 and a downregulation of the protein in DM3. In diabetic groups, the expression of p21CIP1/Waf1 was mainly cytoplasmic but also perinuclear in pachytene spermatocytes and round spermatids. The cytoplasmic localization of p21CIP1/Waf1 may be suggestive of an antiapoptotic role for the protein. The perinuclear localization is probably related to the cell cycle arrest meant for DNA damage repair. Diabetes upregulates p21CIP1/Waf1 signaling in testicular germ cells in association with alteration in p-p53ser315 expression, probably to counteract DNA damage-induced cell death.
Similar content being viewed by others
References
Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature Rev. 2011;11(2):98–107.
Evans JL, Ira ED, Maddux GBA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrine Rev. 2002;23(5):599–622.
Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868.
Marshall SM, Flyvbjerg A. Diabetic nephropathy. In: Holt R, Cockram C, Flyvbjerg A, Goldstein B, eds. Textbook of Diabetes, 4th Edition. Hoboken, NJ: Blckwell Publishing; 2010:599–614.
Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-year medalist study. Diabetes Care. 2011 ;34(4):968–974.
Saboor Aftab SA, Kumar S, Barber TM. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin Endocrinol (Oxf). 2013;78(3):330–337.
Shrilatha B, Muralidhara. Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007;23(4):578–587.
Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K, Siva Narayana T, Ravi Krishna CH. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012;44(suppl l):490–498.
Condorelli RA, Calogero AE, Vicari E, et al. Vascular regenerative therapies for the treatment of erectile dysfunction: current approaches. Andrology. 2013;l(4):533–540.
Narayana K, Yousif MH, El-Hashim AZ, Makki B, Akhtar S, Benter IF. Role of angiotensin II and angiotensin-(l–7) in diabetes-induced oxidative DNA damage in the corpus caverno-sum. Fertil Steril. 2013;100(l):226–233.
Chandrashekar KN, Muralidhara. Evidence of oxidative stress and mitochondrial dysfunctions in the testis of prepubertal diabetic rats. Int J Impot Res. 2009;21(3):198–206.
Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2013; 57(9):1578–1585.
Zhao Y, Zhao H, Zhai X, et al. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol Mech Methods. 2013;23(l):42–47.
Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–1877.
Kushwaha S, Jena GB. Enalapril reduces germ cell toxicity in streptozotocin-induced diabetic rat: investigation on possible mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 2012; 385(2):111–124.
Mallidis C, Agbaje IM, Rogers DA, et al. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int J Androl. 2009;32(4):295–305.
O’Neill J, Czerwiec A, Agbaje I, et al. Differences in mouse models of diabetes mellitus in studies of male reproduction. Int J Androl. 2010;33(5):709–716.
Mallidis C, Agbaje I, O’Neill J, McClure N. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 2009;92(6):2085–2087.
Roy S, Metya SK, Rahaman N, Sannigrahi S, Ahmed F. Ferulic acid in the treatment of post-diabetes testicular damage: relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling. Cell Biochem Funct. 2014;32(1):115-124. doi: 10.1002/cbf.2983.
Jiang X, Zhang C, Xin Y, et al. Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett. 2013;219(l):65–76.
Zhao Y, Tan Y, Dai J, et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200(1–2):100–106.
May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18(53): 7621–7636.
Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic streses. Eur J Biochem. 2001; 268(10):2764–2772.
Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15(2):156–169.
Abbas T, Dutta A. P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–414.
Cmielová J, Rezáčová M. p21Cipl/Wafl protein and its function based on a subcellular localization. J Cell Biochem. 2011; 112(12):3502–3506.
Narayana K, Verghese S, Jacob S. L-Ascorbic acid partially protects two cycles of cisplatin chemotherapy-induced testis damage and oligo-astheno-teratospermia in a mouse model. Exp Toxicol Pathol. 2009;61(6):553–563.
Narayana K. Cisplatin induces duplex 3’ overhangs and 5’ blunt ends in epididymal epithelium in a Bax-dependent manner without any protection from L-ascorbic acid. Eur J Pharmacol. 2010; 641(2–3):238–245.
Narayana K, Al-Bader M, Mousa A, Khan KM. Molecular effects of chemotherapeutic drugs and their modulation by antioxidants in the testis. Eur J Pharmacol. 2012;674(2–3):207–216.
Tsounapi P, Saito M, Dimitriadis F, et al. Antioxidant treatment with edaravone or taurine ameliorates diabetes-induced testicular dysfunction in the rat. Mol Cell Biochem. 2012;369(l–2):195–204.
Broedbaek K, Weimann A, Stovgaard ES, Poulsen HE. Urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine as a biomarker in type 2 diabetes. Free Radic Biol Med. 2011;51(8):1473–1479.
Kyathanahalli C, Bangalore S, Hanumanthappa K, Muralidhara. Experimental diabetes-induced testicular damage in prepubertal rats. J Diabetes. 2013;6(l):48–59.
Rato L, Duarte AI, Tomás GD, et al. Pre-diabetes alters testicular PGCl-α/SIRT3 axis modulating mitochondrial bioenergetics and oxidative stress. Biochim Biophys Acta. 2014;1837(3):335–344.
Koh PO. Streptozotocin-induced diabetes increases the interaction of Bad/Bcl-XL and decreases the binding of pBad/14–3–3 in rat testis. Life Sci. 2007;81(13):1079–1084.
Vendramini V, Cedenho AP, Miraglia SM, Spaine DM. Reproductive function of the male obese Zucker Rats: alteration in sperm production and sperm DNA damage. Reprod Sci. 2014; 21(2):221–229.
Mallidis C, Agbaje IM, Rogers DA, et al. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int J Androl. 2008;32(4):295–305.
Mirzayans R, Andrais B, Scott A, Murray D. New insights into p53 signaling and cancer cell response to DNA damage: implications for cancer therapy. J Biomed Biotechnol, 2012;2012: 170325. doi: 10.1155/2012/170325.
Waterman MJ, Stavridi ES, Waterman JL, Halazonetis TD. ATM- dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19(2):175–178.
Li L, Ljungman M, Dixon JE. The human Cdcl4 phosphatases interact with and dephosphorylate the tumor suppressor protein p53. J Biol Chem. 2000;275(4):2410–2414.
Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36(l):55–62.
Heo JI, Oh SJ, Kho YJ, et al. ERK mediates anti-apoptotic effect through phosphorylation and cytoplasmic localization of p21Wafl/Cipl/Sdi in response to DNA damage in normal human embryonic fibroblast (HEF) cells. Mol Biol Rep. 2011;38(4):2785–2791.
Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704(l–3): 12–20.
Rousseau D, Cannella D, Boulaire J, Fitzgerald P, Fotedar A, Fotedar R. Growth inhibition by CDK-cyclin and PCNA binding domains of p21 occurs by distinct mechanisms and is regulated by ubiquitin-proteasome pathway. Oncogene. 1999;18(30):4313–4325.
Hwang CY, Lee C, Kwon KS. Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21Cipl. Mol Cell Biol. 2009;29(12):3379–3389.
Didenko W, Ngo H, Baskin DS. Early necrotic DNA degradation: presence of blunt-ended DNA breaks, 3’ and 5’ overhangs in apoptosis, but only 5’ overhangs in early necrosis. Am J Pathol. 2003;162(5):1571–1578.
Narayana K, Raghupathy R. DNA damage in lead-exposed hepatocytes: coexistence of apoptosis and necrosis? Drug Chem Toxicol. 2012;35(2):208–217.
Macleod KF, Sherry N, Hannon G, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9(8):935–944.
Javelaud D, Besancon F. Inactivation of p21WAFl sensitizes cells to apoptosis via an increase of both pl4ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J Biol Chem. 2002; 277(40):37949–37954.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kilarkaje, N., Al-Bader, M.M. Diabetes-Induced Oxidative DNA Damage Alters p53-p21CIP1/Waf1 Signaling in the Rat Testis. Reprod. Sci. 22, 102–112 (2015). https://doi.org/10.1177/1933719114533729
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114533729